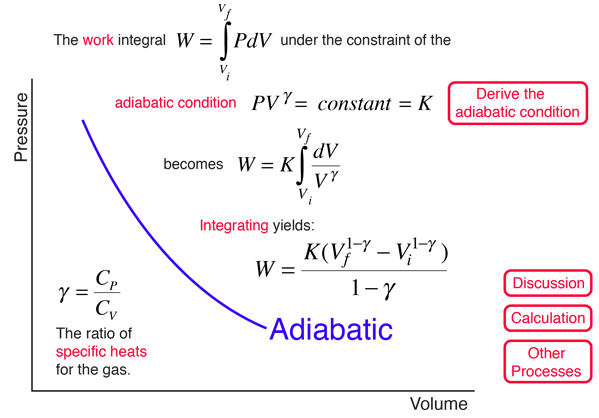
Adiabatic Process
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done. This puts a constraint on the heat engine process leading to the adiabatic condition shown below. This condition can be used to derive the expression for the work done during an adiabatic process.
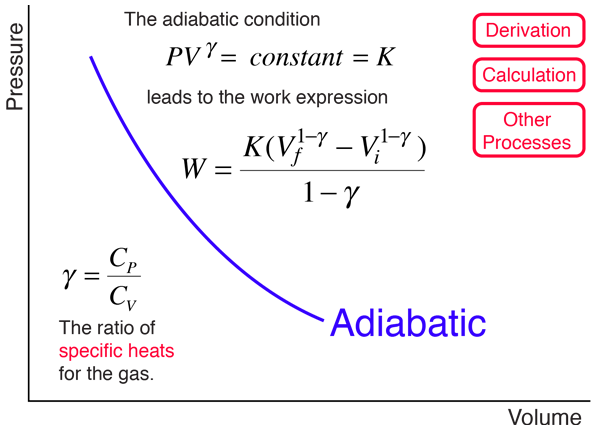
The ratio of the specific heats γ = CP/CV is a factor in determining the speed of sound in a gas and other adiabatic processes as well as this application to heat engines. This ratio γ = 1.66 for an ideal monoatomic gas and γ = 1.4 for air, which is predominantly a diatomic gas.
Heat engine concepts
| HyperPhysics***** Thermodynamics | R Nave |