Active Transport Across Cell Membranes
There are numerous situations in living organisms when molecules move across cell membranes from an area of lower concentration toward an area of higher concentration. This is counter to what would be expected and is labeled "active transport".
There is a very strong tendency for molecules to move from higher concentration to low, just based on thermal energy. Molecules at normal temperatures have very high speeds and random motions. For example, water molecules at 20°C have an effective or rms speed of over 600 m/s or over 1400 miles/hr! This motion from areas of high concentration to low is called diffusion. There are times when membranes are impermeable to some molecules because of their size, polarity, etc. and only the smaller solvent molecules like water molecules will move across the membrane. This is called osmosis, and the tendency to transport the solvent molecules is quantified in terms of osmotic pressure.
If a molecule is to be transported from an area of low concentration to an area of high concentration, work must be done to overcome the influences of diffusion and osmosis. Since in the normal state of a cell, large concentration differences in K+, Na+ and Ca2+ are maintained, it is evident that active transport mechanisms are at work. | 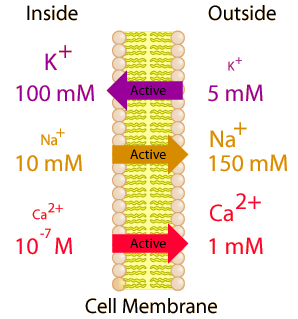 |
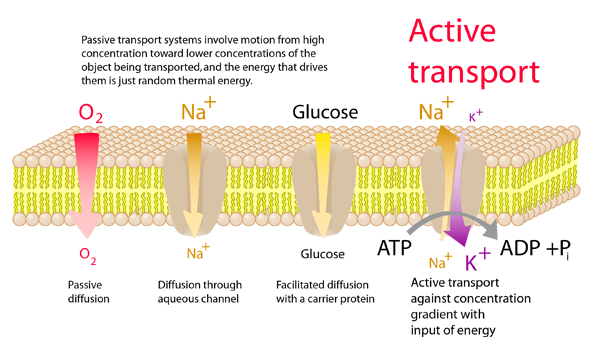
Many crucial processes in the life of cells depend upon active transport. Included in the illustration above is the sodium-potassium pump which is a vital cell process. Active transport mechanisms may draw their enegy from the hydrolysis of ATP, the absorbance of light, the transport of electrons, or coupling with other processes that are moving particles down their concentration gradients.
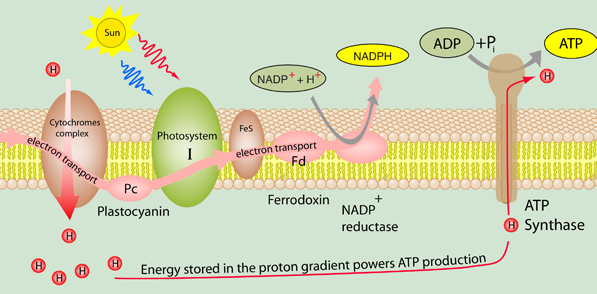
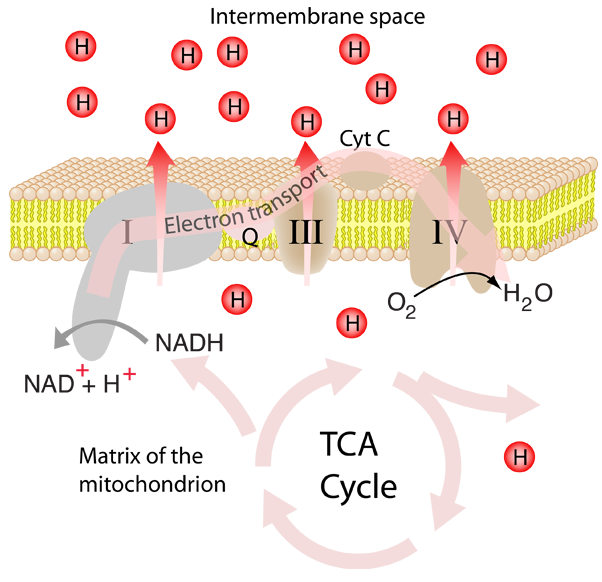
A vital active transport process that occurs in the electron transport process in the membranes of both mitochondria and chloroplasts is the transport of protons to produce a proton gradient. This proton gradient powers the phosphorylation of ATP associated with ATP synthase.
Reference
Karp
Sec 4.7
| HyperPhysics***** Biology | R Nave |