Saturated Vapor Pressure
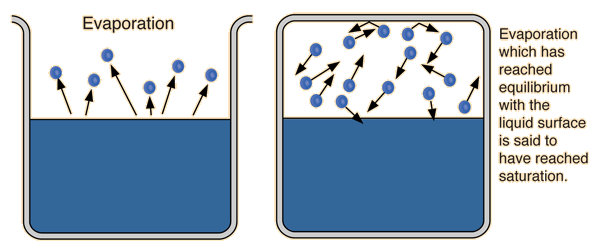
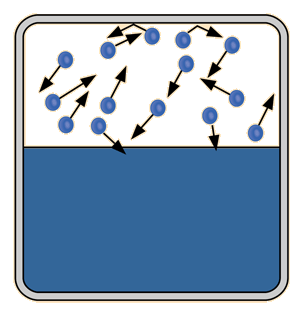
The process of evaporation in a closed container will proceed until there are as many molecules returning to the liquid as there are escaping. At this point the vapor is said to be saturated, and the pressure of that vapor (usually expressed in mmHg) is called the saturated vapor pressure.
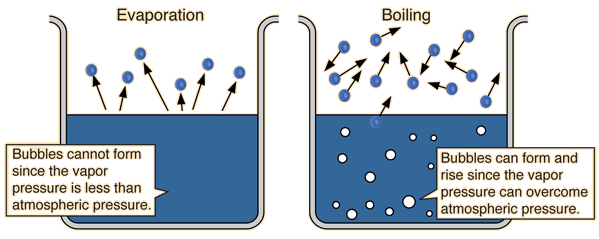
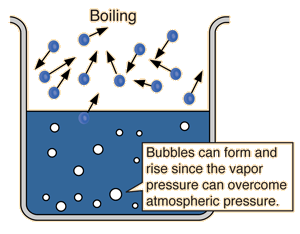
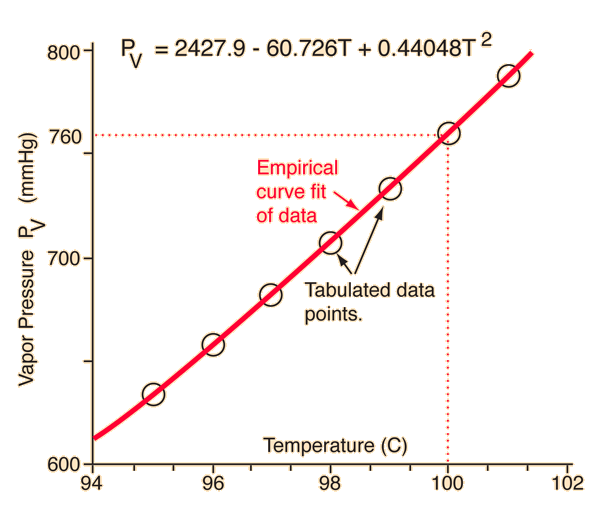
| Since the molecular kinetic energy is greater at higher temperature, more molecules can escape the surface and the saturated vapor pressure is correspondingly higher. If the liquid is open to the air, then the vapor pressure is seen as a partial pressure along with the other constituents of the air. The temperature at which the vapor pressure is equal to the atmospheric pressure is called the boiling point. |
 |
| Table for water | Graph for water |
Kinetic theory concepts
Applications of kinetic theory
Vapor application concepts
| HyperPhysics***** Thermodynamics | R Nave |