Spectrum of Cyanogen, CN
The spectrum of the molecule cyanogen, CN, is of particular interest because it has been observed in space. It was observed in an interstellar cloud in the constellation Ophiuchus, "The Serpent Bearer". The cloud is illuminated from behind by the star z-Ophiuci, making possible the observation of CN absorption lines.
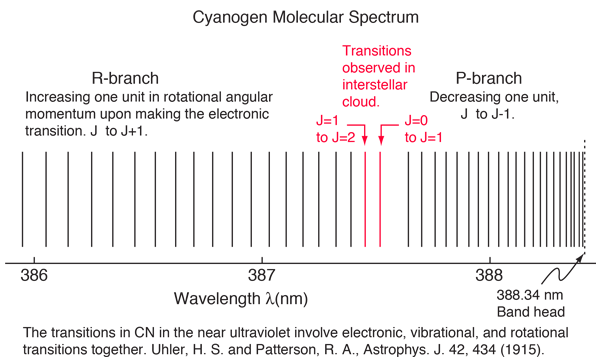
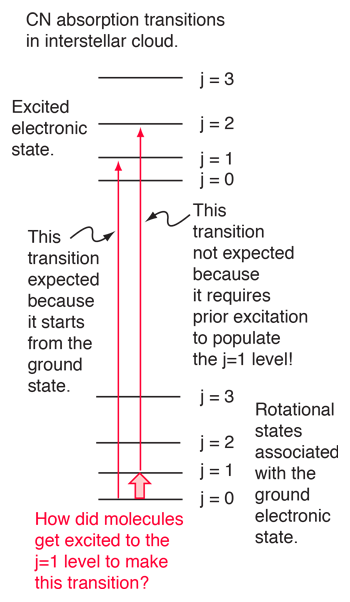 |
An absorption line at 387.5 nm was observed, corresponding to the transition from the j=0 to j=1 rotational state in addition to the larger transition from the ground to excited electronic state. But, surprisingly, another line was observed at a wavelength 0.061 nm shorter and about 1/4 the intensity. Interpreting this transition as the j=1 to j=2 transition required an excitation mechanism, which would involve energy from the surroundings. Using the Boltzmann distribution, a temperature for the environment could be estimated from the relative intensities of the lines. Attributing the excitation to background radiation, the calculation of the required background temperature requires the energy difference (5 x 10-4 eV from the wavelength difference) and the number of ways each state could be formed (2j+1). The intensity ratio is then the population ratio 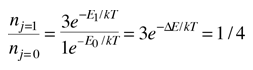 This leads to the temperature 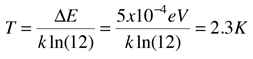 |
About measurements made in the early 40's, Rohlf quotes the noted molecular spectroscopist Gerhard Herzberg as saying "From the intensity ratio of the [CN] lines with [j=0 and j=1] a rotational temperature of 2.3 K follows, which has of course a very restricted meaning." It was not as restricted as he thought -- 25 years later, Penzias and Wilson would demonstrate the existence of the cosmic background radiation at 2.7K, an accomplishment for which they received the Nobel Prize. These early spectroscopists had seen evidence of the cosmic background radiation in the relative intensities of observed spectral lines!
Molecular spectra concepts
Reference
Rohlf
Sec 10-5
| HyperPhysics***** Quantum Physics | R Nave |