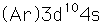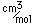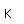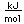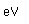Copper
Copper is a red, tough metal with a moderately high melting point. It is an excellent conductor of heat and of electricity and finds extensive use as an electric conductor.
Pure copper is soft and can be drawn into wire or hammered into desired shapes. These shaping processes cause the metal to become hard because the large crystal grains are broken into smaller grains, strengthening the metal. If the copper is subsequently heated (annealed), it can be made soft again.
Copper and zinc are alloyed to make brass, and alloyed with tin to make bronze. Alloys of copper and aluminum are called aluminum bronze.
Copper sulfate (CuSO4.5H20) is a common compound of copper. Copper sulfate is used in copper plating, in fabric printing, and in electric cells. Common names are blue vitriol and blustone. Metallic copper appears in nature in a variety of mineral forms. It appears with magnesium in the carbonate mineral Callaghanite. The carbonate mineral malachite has a rich dark green color which makes it a widely used decorative material. Its companion carbonate azurite has a dramatic blue color.
Cuprite is the mineral form of the oxide Cu2O. Another, rarer, copper oxide is paramelaconite, Cu4O3. Copper forms an oxide with iron called delafossite. An oxide of copper with vanadium is volborthite. An oxide of copper with zinc, vanadium and lead is mottramite.
Copper forms the sulfides covellite, CuS, chalcocite, Cu2S, and digenite, Cu9S5. With iron it forms chalcopyrite, CuFeS2, with antimony it forms chalcostibite, CuSbS2 and with arsenic itforms chalcophyllite. Copper also forms a sulfide with bismuth called emplectite, CuBiS2. It forms a sulfide with antimony and iron called tetrahedrite.
Sulfides with iron include bornite, Cu5FeS4, and cubanite, CuFe2S3. A sulfide with arsenic is called enargite. Bournonite is a sulfide with copper, lead and antimony. Tennantite is a sulfide which contains copper, arsenic, iron and antimony. Lead, copper and iron join in the sulfide betekhtinite. Germanite is a sulfide which contains copper, germanium and iron. Stannite is a sulfide with tin and copper. Copper with silver and antimony form the sulfide polybasite.
It occurs in a sulfate form in antlerite, brochantite, connellite, cyanotrichite and devilline. Copper appears with sodium in the sulfate krohnkite which exhibits a dramatic blue color.
Copper appears with lead in caledonite. Copper, cobalt and nickel join in the sulfide carrollite.
A striking green color is exhibited by the mineral bayldonite which combines copper, zinc and lead with an arsenate group. Other green copper minerals are the copper arsenates olivenite, Cu2AsO4(OH) and tyrolite. Another copper arsenate is clinoclase. Duftite is an arsenate mineral of lead and copper. Liroconite is an arsenate mineral of aluminum and copper with a blue color.
Copper silicate in the mineral dioptase also exhibits a green color. Dioptase is sometimes accompanied by a rarer silicate of copper, plancheite. Copper silicate in the mineral dioptase also exhibits a green color.
Another silicate of copper is shattuckite with a blue color.
Other copper containing minerals are boleite and cumengite which contain chlorine. A chloride of copper and lead is diaboleite. Another chloride of copper is atacamite.
Copper appears as a phosphate in cornetite and turquoise. It also forms the phosphate pseudomalachite. Copper forms the phosphate mineral libethenite. Copper appears with uranium in the phosphate mineral metatorbernite. Copper appears with zinc in the phosphate mineral veszelyite. The phosphate mineral tsumebite contains lead and copper and shows a green color.
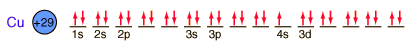
Copper is one of the few exceptions to the general order of filling of electron orbitals, filling all ten 3d states before it fills the second 4s state.
|