The Sodium Doublet
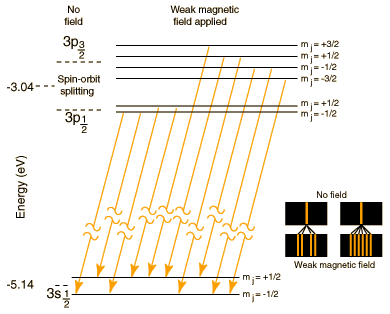
 | The well known bright doublet which is responsible for the bright yellow light from a sodium lamp may be used to demonstrate several of the influences which cause splitting of the emission lines of atomic spectra. The transition which gives rise to the doublet is from the 3p to the 3s level, levels which would be the same in the hydrogen atom. The fact that the 3s (orbital quantum number = 0) is lower than the 3p (l=1) is a good example of the dependence of atomic energy levels on angular momentum. The 3s electron penetrates the 1s shell more and is less effectively shielded than the 3p electron, so the 3s level is lower (more tightly bound). The fact that there is a doublet shows the smaller dependence of the atomic energy levels on the total angular momentum . The 3p level is split into states with total angular momentum j=3/2 and j=1/2 by the magnetic energy of the electron spin in the presence of the internal magnetic field caused by the orbital motion. This effect is called the spin-orbit effect. In the presence of an additional externally applied magnetic field, these levels are further split by the magnetic interaction, showing dependence of the energies on the z-component of the total angular momentum. This splitting gives the Zeeman effect for sodium. |
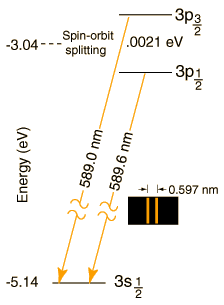
The magnitude of the spin-orbit interaction has the form μzB = μBSzLz. In the case of the sodium doublet, the difference in energy for the 3p3/2 and 3p1/2 comes from a change of 1 unit in the spin orientation with the orbital part presumed to be the same. The change in energy is of the form
ΔE = μBgB = 0.0021 eV
where μB is the Bohr magneton and g is the electron spin g-factor with value very close to 2. This gives an estimate of the internal magnetic field needed to produce the observed splitting:
μBgB = (5.79 x 10-5 eV/T)2B = 0.0021 eV
B = 18 Tesla
This is a very large magnetic field by laboratory standards. Large magnets with dimensions over a meter, used for NMR and ESR experiments, have magnetic fields on the order of a Tesla.
| More on sodium spectrum | Sodium Zeeman effect |
Other spectra
References
Thornton & Rex
Sec 9.2
Serway, Moses, Moyer
Sec 8.3
| HyperPhysics***** Quantum Physics | R Nave |