Corrosion as an Electrochemical Process
A piece of bare iron left outside where it is exposed to moisture will rust quickly. It will do so even more quickly if the moisture is salt water. The corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact with the metal, oxidizing the iron.
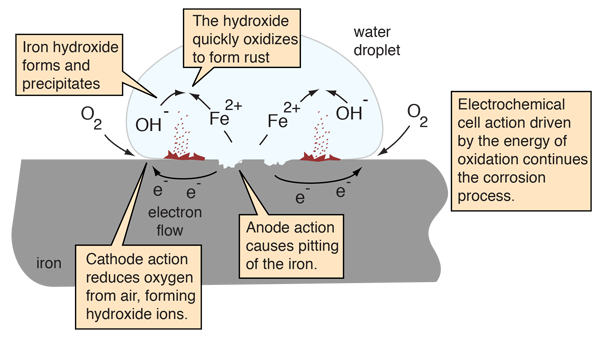
Considering the sketch of a water droplet (after Ebbing), the oxidizing iron supplies electrons at the edge of the droplet to reduce oxygen from the air. The iron surface inside the droplet acts as the anode for the process
Fe(s) -> + Fe2+(aq) + 2e-
The electrons can move through the metallic iron to the outside of the droplet where
O2(g) + 2H2O(l) + 4e- -> 4OH-(aq)
Within the droplet, the hydroxide ions can move inward to react with the iron(II) ions moving from the oxidation region. Iron(II) hydroxide is precipitated.
Fe2+(aq) + 2OH-(aq) -> Fe(OH)2(s)
Rust is then quickly produced by the oxidation of the precipitate.
4Fe(OH)2(s) + O2(g) -> 2Fe2O3 •H2O(s) + 2H2O(l)
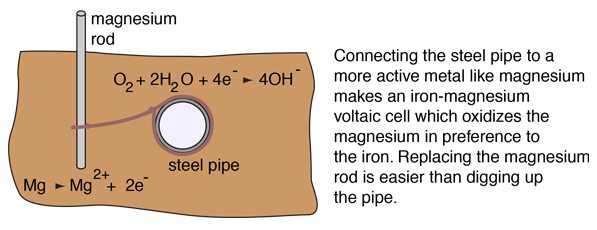
The rusting of unprotected iron in the presence of air and water is then inevitable because it is driven by an electrochemical process. However, other electrochemical processes can offer some protection against corrosion. For magnesium rods can be used to protect underground steel pipes by a process called cathodic protection.
| Cathodic protection from corrosion |
Oxidation/
Reduction concepts
Electrochemistry concepts
Reference
Hill & Kolb
Ch 8
Ebbing
Ch 19
| HyperPhysics***** Electricity and Magnetism ***** Chemistry | R Nave |