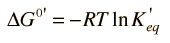Gibbs Free Energy and the Spontaneity of Chemical Reactions
The change in Gibbs free energy associated with a chemical reaction is a useful indicator of whether the reaction will proceed spontaneously. Since the change in free energy is equal to the maximum useful work which can be accomplished by the reaction
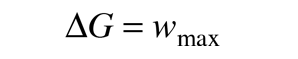
then a negative ΔG associated with a reaction indicates that it can happen spontaneously. This is consistent with the usual chemistry convention of treating work done by the system as negative work. Most common reactions can be assessed for spontaneity under standard conditions by looking up the associated thermodynamic quantities for each of the reactants and products. For non-standard conditions one can make use of the the expression for ΔG in terms of the other thermodynamic potentials
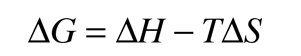
The change in free energy ΔG for a reaction can be expressed in terms of the equilibrium concentrations of the reactants and products. It is convenient to express ΔG for any reaction in terms of its value under standard conditions where values have been tabulated. For a reaction represented as
the change in free energy can be expressed as
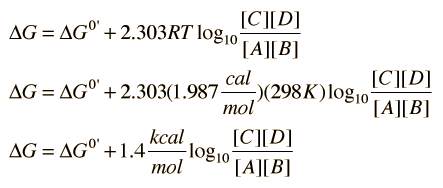
Where ΔG0' is the standard free energy change and it is presumed that the temperature is 25°C = 298K.
| Example of hydrolysis of ATP |
Electrochemistry concepts
Reference
Ebbing
Ch 18
Karp
Ch 3
| HyperPhysics***** Electricity and Magnetism ***** Chemistry | R Nave |