Transition Elements
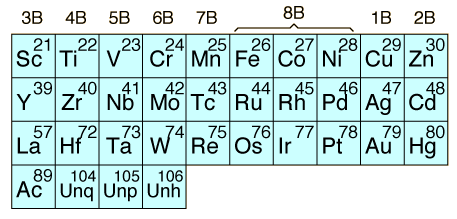
The transition elements are those elements having a partially filled d or f subshell in any common oxidation state. The term "transition elements" most commonly refers to the d-block transition elements. The 2B elements zinc, cadmium and mercury do not strictly meet the defining properties, but are usually included with the transition elements because of their similar properties. The f-block transition elements are sometimes known as "inner transition elements". The first row of them is called the lanthanides or rare earths. The second row consists of the actinides. All of the actinides are radioactive and those above Z=92 are manmade in nuclear reactors or accelerators.
The general properties of the transition elements are
- They are usually high melting point metals.
- They have several oxidation states.
- They usually form colored compounds.
- They are often paramagnetic.
The transition elements include the important metals iron, copper and silver. Iron and titanium are the most abundant transition elements. Many catalysts for industrial reactions involve transition elements.
| Chemistry of the Elements |
Periodic Table
Chemistry concepts
Reference
Ebbing
Ch 23
| HyperPhysics | R Nave |