What Causes Electron Energies to Depend Upon the Orbital Quantum Number?
From the Bohr model or the hydrogen Schrodinger equation, the solution for the electron energy levels gives:
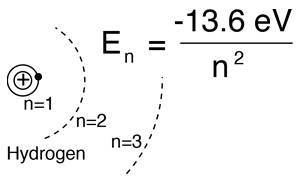
|
This fits the hydrogen spectrum unless you take a high resolution look at fine structure or the structure produced by external magnetic fields (Zeeman effect), etc. |
Hydrogen-like atoms such as lithium and sodium might be expected to exhibit similar energy levels. They consist of closed shells with a single electron outside. Envisioning a Bohr-type shell structure with just a single electron in the outer shell, the net charge inside that shell is just one net positive charge. This leads to the following expectation:
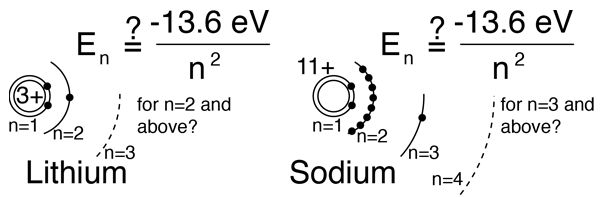
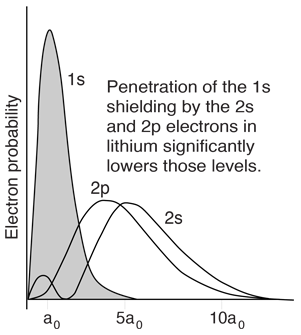
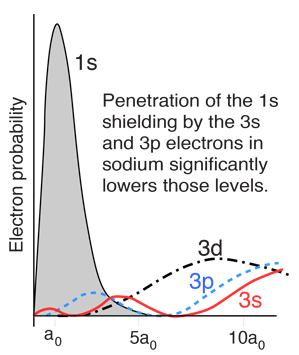
However, when data from spectra are used to build energy level diagrams for these atoms, a strong orbital dependence of the energy is found for the electrons of low angular momentum as shown below.
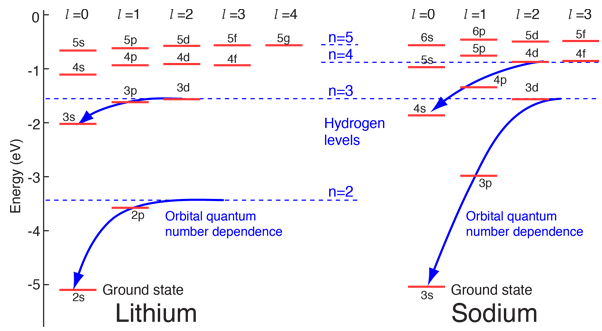
What is the origin of the orbital quantum number dependence?
Schrodinger equation concepts
Hydrogen concepts