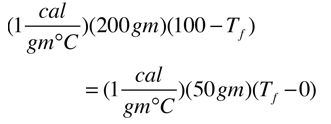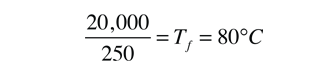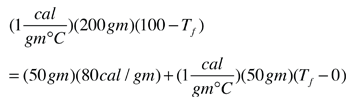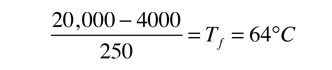Cooling a Cup of Coffee
You have a 300 gram cup of coffee at 100 °C, too hot to drink. How much will you cool it by forcing 50 gm to evaporate, leaving 250 gm?
Heat lost by coffee = Heat of vaporization
-Qcoffee = Qvaporization
-cmcΔTcoffee = mvLv
(1 cal/gm°C)(250gm)(100-Tf)=(540cal/gm)(50gm)
25,000 - Tf = 27,000
But this can't be right because it gives a negative temperature (-8°C)
and the specific heat equation Q = cmΔT is valid only so long as a
phase change is not encountered, so we can't pass 0°C with this
equation. If 25000 calories are extracted, we have cooled the coffee to 0°C
but still have 2000 cal to remove. This will freeze some of the coffee:
(2000 cal)/(80 cal/gm) = 25 gm frozen at 0°C
|

| |
|
Index
Heat transfer concepts
Heat transfer examples |