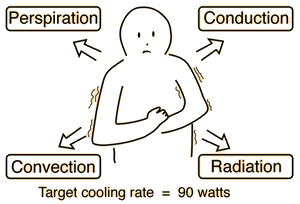
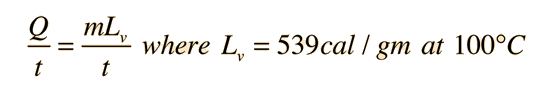Perspiration Cooling of Body
 For 600 gm/day perspiration,
|
When the ambient temperature is above body temperature, then radiation, conduction and convection all transfer heat into the body rather than out. Since there must be a net outward heat transfer, the only mechanisms left under those conditions are the evaporation of perspiration from the skin and the evaporative cooling from exhaled moisture. Even when one is unaware of perspiration, physiology texts quote an amount of about 600 grams per day of "insensate loss" of moisture from the skin. The cooling effect of perspiration evaporation makes use of the very large heat of vaporization of water. This heat of vaporization is 540 calories/gm at the boiling point, but is even larger, 580 cal/gm, at the normal skin temperature. |
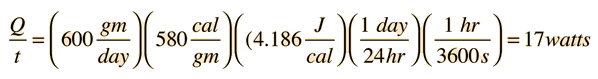
As part of the physiological regulation of body temperature, the skin will begin to sweat almost precisely at 37°C and the perspiration will increase rapidly with increasing skin temperature. Guyton reports that a normal maximum perspiration rate is about 1.5 liters/hour, but that after 4 to 6 weeks of acclimatization in a tropical climate, it can reach 3.5 liters/hr! You would have to just sit around drinking constantly, just to keep from getting dehydrated! That maximum rate corresponds to a maximum cooling power of almost 2.4 kilowatts!
| Heat transfer by vaporization | Evaporation cooling |
| Modeling the cooling of the human body |
Heat transfer concepts
Heat transfer examples
Reference
Guyton
Ch. 47
| HyperPhysics***** Thermodynamics | R Nave |