Bangor and the University College of North Wales
1966-67

Bangor, North Wales is located on the Menai Strait and is the home of the University College of North Wales, where Rod did a post-doc in the Department of Chemistry under the direction of Professor John Sheridan. You can see the typical architecture of Welsh towns with long rows of attached houses that follow the landscape. In the center is the square tower of Bangor Cathedral. The residential area of Bangor extends up the side of Bangor Mountain.

The main building of the Univerity College of North Wales was set up on a ridge above most of the other buildings, and was commonly and I suppose affectionately referred to as "Top Col".

The square, tall building in the center of the picture is the Department of Chemistry building where Rod worked in the microwave spectroscopy laboratory. The hospital where Brenda worked as a nurse is the building immediately behind the Chemistry Building, so that was convenient.
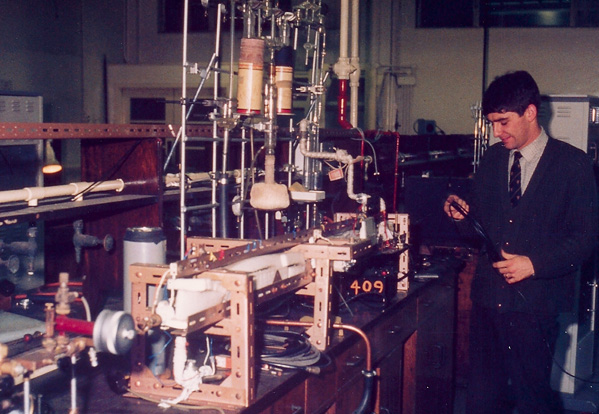
Chemistry graduate student John Griffith works with the glass vacuum system which is used to let the gases to be studied into the copper waveguide. There they were subjected to microwave energy to measure the frequencies at which the rotating molecules absorbed microwaves. From those absorption frequencies could be calculated bond lengths, angles, and other structural information about molecules, a "molecular microscope".

|
Above, Valerie Williams adjusts the glass vacuum system which allows the gas to be studied into the experimental waveguide chamber. Graham Williams and his wife Valerie were both graduate students in chemistry. They were working on Ph. D. theses in microwave spectroscopy. Graham is working with some of the electronics which produces and controls the microwave radiation to send down the waveguide and through the sample gas. |
 |
Retrospective on Rod's research at University College of North Wales, Bangor
Since I didn't record much about my work in research in the Chemistry Department of the University College of North Wales at the time, these comments are just general reflections looking back after many years. I enjoyed the work with the graduate students and appreciated the opportunity provided by Professor John Sheridan. Since they also lived in Beaumaris, we had occasion to get to know Mrs Sheridan and their sons as well during the year. Besides the work with the students, I had my own research projects during the year. The most productive of the projects was with the molecule SPF2H which has the ponderous name hydro-thio-phosphoryl-diflouride. The structures of most small molecules had already been determined at this point in history since the science of microwave spectroscopy had been pursued since World War II days. The reason that the structure of this molecule had not been previously determined was that it had never been synthesized. But Professor Sheridan had a research collaborator in Canada who had just managed to synthesize and purify it. One of the reasons that it had never been synthesized was that it was quite unstable and in fact explosive on contact with air!
Professor Sheridan persuaded his collaborator to send to us a sample - a tiny sample in a glass ampule which was then carefully packed and sent across the Atlantic to Wales from Canada. I was able to get that sample into the glass vacuum system shown above by immersing it in liquid nitrogen to solidify the sample. The ampule had been made with a small end which I could insert into a flexible hose which was then attached to the vacuum system and evacuated to a high vacuum condition. With the sample still frozen, I then snapped off the pointed end of the glass ampule. To transfer the SPF2H to the vacuum system I set up another standard glass container about 3 cm in diameter with a glass stopcock and immersed that container in liquid nitrogen, continually pumping on the system with the vacuum pump to maintain a high vacuum. Then the container which had the SPF2H was allowed to warm up slowly and the evaporating compound was then trapped by the liquid nitrogen cold trap. Once the transfer was complete, the stopcock on the storage container was closed and that became my working sample for the better part of a year.
To study the SPF2H I would again immerse the end of the storage container in liquid nitrogen to trap out the sample, open the stopcock, and then lower the liquid nitrogen from the container while monitoring the pressure. When the pressure started going up, I closed the stopcock. If I didn't have enough gas pressure for study, I could repeat the process. The gas sample was then allowed to enter the long copper waveguide, probably about 10 feet long. Then I would send precisely measured frequencies of microwave radiation down the waveguide to measure the frequencies at which the molecules absorbed the microwave energy. The absorption of the specific microwave frequencies occurred because those frequencies caused a transition between the quantum energy levels of molecular rotation of the molecules. A precise energy difference between two quantum rotational levels of the molecule gave rise to the absorption of microwave photons of quantum energy precisely equal to that energy level separation.
To actually detect and measure the frequency and amount of absorption of the microwaves, another step was necessary. The absorption at a given frequency was actually only a very tiny fraction of the microwave energy coming down the waveguide, so measuring it as a fraction of the energy absorbed was quite impractical. We used a modulation scheme called Stark modulation. With a flat electrode placed in the center of the waveguide, we could impose an electrical square wave at about 100 kiloHertz on the gas in the waveguide. This amounted to turning a high electric field on and off at 100kHz. The electric field changed the frequency at which the molecule absorbed microwave energy, an effect called the Stark effect. That meant that if you had a frequency at which the gas absorbed energy and the field was switched on, it would no longer absorb. That is, you could switch the absorption on and off at 100 kHz. This produced a 100 kHz modulation of the microwave energy at a detector crystal at the end of the waveguide which could be measured with far greater sensitivity than the measurement of changes in the overall power level. Such modulation techniques are used in many types of spectroscopy experiments to provide the necessary sensitivity for the study of quantum absorptions.
When I first introduced SPF2H into the waveguide and swept through a range of microwave frequencies, the results were very exciting! There was an abundance of strong microwave absorption lines, and they showed strong Stark lobes. Since the imposition of the electric field on the gas changed the separation between the rotational energy levels, it caused the gas to absorb at a different frequencies, and that absorption gave a 100 kHz modulation signal that was 180 degrees out of phase with the zero field absorption. When plotted out on a chart recorder, I saw strong positive zero-field lines and then several negative signals for the "field-on" transitions. This is because the Stark effect breaks up a single pair of absorbing energy levels into several levels, depending upon the rotational quantum numbers of the levels. I could gradually increase the strength of the modulating electric field and watch the "Stark lobes" move outward from the zero field line. The entire process of absorption could be modeled by a quantum mechanical calculation, so the observation of the frequency of an absorption and the number of Stark lobes could be compared with the model to identify which rotational quantum levels of the molecule were being observed.
Once the molecular rotational quantum numbers associated with the particular absorption frequencies were identified, then that information could be used to calculate the moments of inertia of the molecule, and with sufficient data on the moments of inertia of the molecule the actual structure of the molecule could be calculated. That is, the actual numbers for the bond lengths and angles of the molecule can be determined from the microwave absorption frequencies once they are identified with their specific quantum levels. That was the excitement of that study of SPF2H! There was the potential that I could actually determine to three decimal places the bond lengths and angles of this molecule which had only been synthesized a matter of months before I received the sample.
Most researchers in microwave spectroscopy at this time in history never got the opportunity to solve a complete structure, so this was indeed an exciting research possibility for me. But much work lay ahead. The molecular rotational energy levels for the common isotopic species of the molecule were identified fairly quickly and the three moments of inertia were determined with precision. But examination of the molecule shows three different bond lengths and three different angles required to determine the actual geometry of the molecule. This implies that I needed six independent experimental parameters to completely determine the structure. A powerful part of the technique of microwave spectroscopy is the measurement of different isotopic species of the molecule. Because the molecular structure is determined by the electric forces between the atoms and not their masses, at least to a high degree of accuracy, then the molecule SPF2D in which the common hydrogen atom is replaced by its heavy isotope deuterium will have the same structure. Professor Sheridan asked his Canadian colleague whether he could synthesize the molecule with deuterium instead of light hydrogen, and he did so promptly and sent us a sample. It looked like I had the green light toward determining the complete structure of this molecule!
I promptly introduced the deuterated species of the molecule into the spectrometer and started rapidly collecting data. From having solved the normal species model, I had a good indication of where to look for the important absorption lines of the deuterated species, so the work was going quickly toward determining the moments of inertia of the deuterated molecule. That would give me six parameters to work toward the determination of the six-parameter structure of the molecule. But then disaster struck! We came into the lab one morning to find that the glass vacuum system had exploded and there was glass everywhere. Fortunately, no one was in the lab when the explosion occurred, so there were no injuries, but it destroyed my deuterated sample. We of course knew very well about the explosive nature of this compound on contact with air and had carefully protected the system to keep it protected from air leaking into the system. But we presumed that one of the many stopcocks associated with the glass vacuum system had sprung a leak and the presence of air in the system led to the explosion.
Having planned to take much more data on the deuterated species to give precision to the moment of inertia, I thought I was defeated and talked to Professor Sheridan about asking his Canadian colleague to make another deuterated sample. But, understandably, he just couldn't bring himself to do it. His response, in his strong British accent, was "I just can't ring him up and tell him that we dropped it on the floor!" So he sent me back to the lab to see if I had enough data already to solve for the needed moments of inertia. After a few days of work, I found to my great relief that I did have enough data to calculate the three moments of inertia of the deuterated molecule. I would have liked to have had more precision, but in fact it was quite good data.
With my six experimentally determined parameters I started the process of calculating the six necessary bond lengths and angles to specify the structure of SPF2H. That brought me to the second great shock in this experimental adventure. I had six parameters from the experiment, and with six equations and six unknowns I should be able to solve for the six parameters of the molecular structure, although clearly it was a pretty laborious algebra problem. But six parameters could allow me to solve for the six structural parameters only if they were six independent experimental parameters! But the molecule SPF2H has a plane of symmetry between the two fluorine atoms. That means that the two moments of inertia in the SPH plane are related to the third moment of inertia by the perpendicular axis theorem - they are not independent! So the data failed to give me the six independent equations necessary to solve for six structural parameters.
Obtaining additional experimental parameters would require another isotopic species to be able to calculate the complete molecular structure. The most attractive possibility was to have a sample of 34SPF2H rather than the common 32SPF2H . But that would again put us in the position of asking our Canadian benefactor to remake the molecule with sulfur-34 instead of sulfur-32, and that was a more difficult synthesis than just replacing a hydrogen with a deuterium. After two great shocks on the course of this experimental journey, there was a ray of light that occurred to us. The experimental spectrum of 32SPF2H was extremely strong, so we began to consider the possibility that we could observe the spectrum of 34SPF2H in natural abundance! About 3% of natural sulfur is the 34S isotope! Even though the spectral lines for the 34S species would be only about 1/33 as strong, tiny peaks among towering peaks of the normal species, still I had the advantage of knowing fairly precisely where to look for them. Since I already had the moments of inertia for the normal and deuterated species, I could project a very good model of the 34S species. To my great relief, I was able to find enough of them to calculate the moments of inertia of the third species, giving me nine experimental parameters. Even with the plane of symmetry problem, that gave me six independent parameters and I was able to solve for the complete structure.
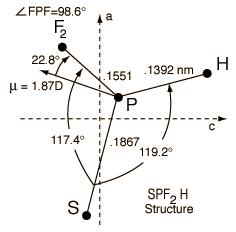 | I am grateful for having had the experience of contributing the complete structure, bond lengths and angles, for a molecule. The great precision of microwave spectroscopy is the most accurate way to determine such structures. That research contributed to chemical understanding, particularly when combined with the structures of other similar molecules. But since this molecule is hard to synthesize and is violently explosive on contact with air, not many people are going to encounter it. My standard line over the years has been "I am the world expert on the structure of hydro-thio-phosphoryl-diflouride, but hardly anyone ever asks me about it!" |
| Adjusting to the stamps, currency, and AC outlets. |
1966