Upon the Stability of the Deuteron Hangs the Future of the Early Universe
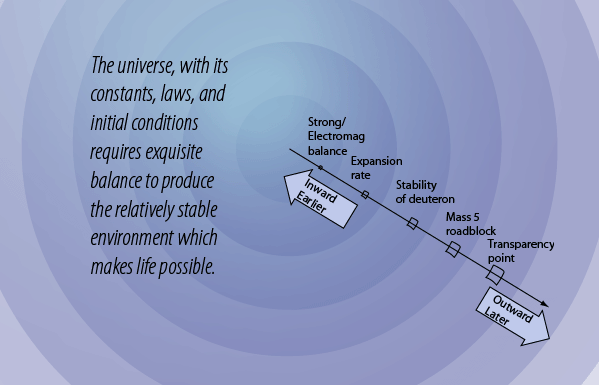
Our understanding of the hot early universe is that at some stage the universe was composed of about equal numbers of protons and neutrons plus about equal numbers of electrons and positrons. One reason that we suppose this is that at temperatures in the neighborhood of 1011 K , which would have been about 0.02 seconds after the big bang according to Weinberg's model, there is enough thermal energy to convert protons into neutrons and vice versa. There is also enough thermal energy to produce electron-positron pairs. At this stage this energy was primarily in the form of the intense radiation field associated with this elevated temperature - it would subsequently cool to form the cosmic background radiation that we observe today.
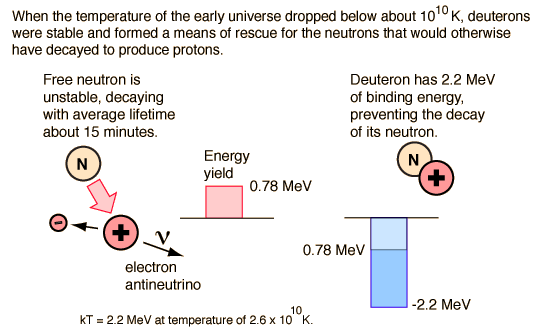
However, with the expansion and cooling of the early universe, it quickly reaches the point at which the available thermal energy is not sufficient to make the step upward in mass energy of 1.29 MeV from the proton to the neutron. Adding to the precariousness of this situation is the fact that the neutron is unstable when isolated as a single particle and decays with a mean lifetime of about 15 minutes. So the neutrons begin decaying and there is an excess of protons over neutrons. If allowed to persist, all the neutrons would decay to protons, and no nuclei other than hydrogen could exist in the universe. That is to say, there would be no universe as we know it.
Fortunately for the universe, there is an escape from this scenario. Neutrons can collide with protons and bind to them with the nuclear strong force, forming deuterium. Deuterium is an isotope of hydrogen. Because of the nature of nuclear binding energy, the mass of the deuteron is slightly less than the sum of the proton plus neutron masses and it is therefore stable against decay into a neutron and proton. The neutron is stable against decay when it is bound into a deuteron. But its margin of stability is small, one of the fine balances of nature, and without its stability there would be no universe. Deuterium is not stable until the temperature of the universe drops to about 109K where the thermal energy is 0.13 MeV compared to the 2.2 MeV binding energy of the deuteron. If 0.13 MeV is the average kinetic energy, then very few will be found to have enough energy to break apart a deuteron. According to Weinberg's timeline, this stable rescue of the neutrons is at about 3 minutes into the age of the universe.
This "window of creation" points back to the previous one. If the expansion rate were slower and the temperature stayed up beyond the stability point of the deuteron for longer, then we would have fewer neutrons because their decay as free neutrons would be proceeding. Considering the fact that the decay of a neutron to a proton releases 1.29 MeV of energy, one would expect it to proceed much more rapidly than a 15 minute average lifetime. But the length of the lifetime allowed enough of them to survive to be bound into deuterium and contribute to our present universe.
| After making deuterons, why don't the deuterons keep combining into heavy elements? |
| Deuterium abundance |
HyperPhysics  | R Nave |