Nitrogen Fixation
 | Nitrogen fixation in the roots of plants such as legumes is the only biological pathway for providing nitrogen fo the essential life process such as making amino acids, the building blocks for proteins.
The conversion of atmospheric N2 to ammonia, NH3, uses a process in the nodules on the roots of leguminous plants which involves enzymes with the metals iron and molybdenum (sometimes vanadium).
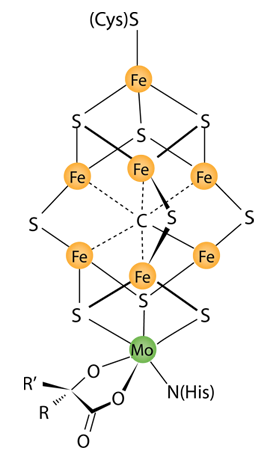
The diagram at left is to provide an example of the type of complexes in biology which make use of metal atoms. Iron is common in such applications, but in this case molybdenum is critical in the nitrogen fixation process. This complex is described as a MoFe cofactor in a MoFe protein.
|
The actual breaking of the strong N2 triple bond and production of NH3 and other nitrogen compounds is accomplished by bacteria in symbiotic relationship with plant groups such as legumes. The types of microorganisms are called diazotrophs. The enzymes involved are called nitrogenases.
Looser non-symbiotic relationships (called associations) occur between diazotrophs and plants such as rice where nitrogen fixation takes place on the roots.
The overall reaction for the conversion to ammonia enabled by the nitrogenase enzyme may be written:
N2 + 16 ATP + 16 H2O + 8e- + 8H+ --> 2NH3 + H2 + 16ADP + 16Pi
This can be seen to be an energy-intensive process by the requirement of 16 of the energy-rich ATP molecules. The conversion of N2 into ammonia occurs at a metal cluster called FeMoco, an abbreviation for the iron-molybdenum cofactor. The mechanism proceeds via a series of protonation and reduction steps wherein the FeMoco active site hydrogenates the N2 substrate.
Some plants that contribute to nitrogen fixation are members of the legume family: kudzu, clover, soybean, alfalfa, lupin and peanut. They contain the symbiotic rhizobia bacteria within nodules in their root systems. These nodules limit exposure to oxygen, which rapidly decomposes the nitrogenase enzymes. Nitrogen fixation can occur in decomposing wood by diazotrophic bacterial communities. This can enrich the nitrogen content of the wood, enabling deadwood decomposition by fungi.
A large amount of nitrogen fixation occurs in the oceans, perhaps half. The cyanobacteria in the seas are a major source of such production of nitrates.
|
Index
Reference
Nitrogen fixation wiki
Denton, Miracle of the Cell
Chap 6 |