Seawater
Water has great abundance on the Earth, and of that abundance about 97% is sea water. Sea water contains about 3.5% by weight of salt (sodium chloride). The salinity does vary, and the combination of salinity and temperature has a major influence on ocean currents and behavior. Salinity is a crucial property of the seas and is widely measured. Among the variouse ways to characterize the average salinity are 35 ppt (parts per thousand), 35 psu (practical salinity units). The typical range of salinity is about 33 to 37 ppt. About 70% of the Earth is covered with water. The salt in that water would build a 180 mile high, 1 mile thick wall around the equator according to the Windows to the Universe website.
| There are other salts dissolved in sea water, with ordinary sodium chloride constituting about 90% of the dissolved salt. The table at left is given by the Windows on the Universe website. It is not clear how you reach charge neutrality with these numbers. Another item of information from this site was that deep ocean water was at about 3°C and salinity 34-35 ppt. Seawater of average salinity 35 ppt freezes at -1.94°C (28.5°F). The salinity of the water is measured with a CTD instrument (Conductivity, Temperature, Depth). The conductivity is used to calculate the salinity in conjunction with the temperature and depth (pressure) measurements. | ||||||||||||||
Seawater has an average density of 1.027 gm/cm3, but this varies with temperature and salinity over a range of about 1.020 to 1.029. The density is typically indicated by a density index σ which is the departure of the density from 1.000 multiplied by a thousand. | 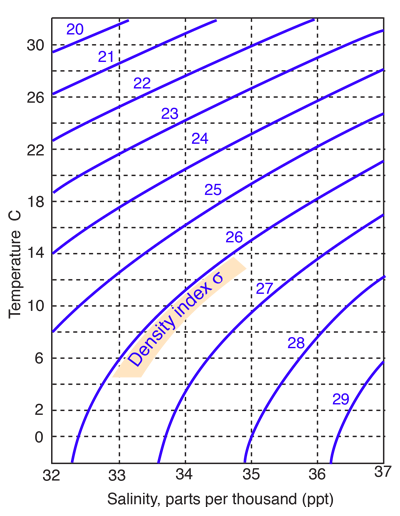
|
| Water available on Earth |
Water concepts
Chemistry concepts
Reference
Windows to the Universe
| HyperPhysics*****Chemistry | R Nave |