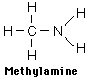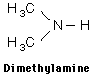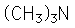Amines
Amines are organic compounds that contain nitrogen and are basic. The general form of an amine is shown in Lewis form. R represents an alkyl group, but either or both of the hydrogens may be replaced by other groups and still retain its class as an amine. |
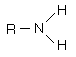
|
Examples showing different ways to show condensed formuli are:
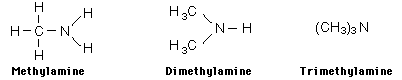
The fact that amines tend to have foul odors is emphasized by the names of two amines: cadaverine and putresine.
Amines are basic compounds with strong odors. The odor of amines is often described as "fishy" since the odor of raw fish comes from the amines contained. Sometimes even "foul smelling" is an understatement, and two of the amines produced in decaying flesh have suggestive names (cadaverine and putresine).
Despite this foul reputation, the amines are essential to life as constituents of amino acids. They occur in drugs and vitamins, and are essential starting materials for many synthetic processes. The aromatic amine aniline is the basis for the synthesis of a whole class of synthetic dyes. Synthetic amines such as benzedrine have medical applications.
| Hydrocarbon derivatives |
Carbon compounds
Chemistry concepts
Reference
Shipman, Wilson, Todd
Sec 15.4
| HyperPhysics*****Chemistry | R Nave |