The division into fermions and bosons
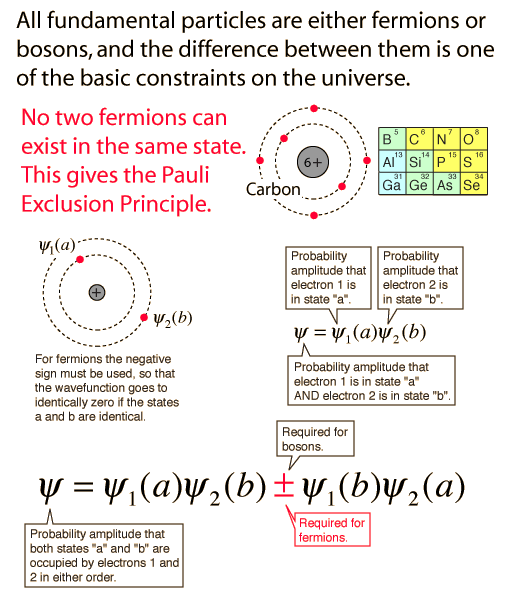
The electon is one of the particles that we consider to be an elementary particle, i.e., one that is not made up of some other kind of particles collectively. It has a property called electron spin and is assigned a spin value s=1/2. Particles with spin 1/2 or any non-integer spin are called fermions. Particles with integer spin are called bosons.
This may not sound exciting, but it is in fact one of the essential keys or "windows" to the existence of our universe and the fundamental structure of matter. When the basic quantum mechanical treatment of electrons is done, we find that the wavefunction for a system in which two electrons are in exactly the same state is identically zero! That is, no two electrons can occupy the same energy state. The same applies to protons and neutrons which also have spin 1/2 and are therefore fermions. All the particles of nature are divided into fermions and bosons. Many bosons can gather into the same state to produced collections called Bose-Einstein condensates that have extraordinary properties, but it is fermions which are part of the story of how the current universe came to be.
The rule that no two electrons can occupy the same energy state is familiar in chemistry as the Pauli exclusion principle, essential for building up atoms and the periodic table as we know it. That is, the division into fermions and bosons and the Pauli exclusion principle form a window or constrain which was necessary to have a universe. This principle is fundamental to the universe, and had to be in place for the creation of atoms.
| The equality of the number of protons and electrons |
HyperPhysics  | R Nave |