Metabolism
If you burn wood, you liberate energy stored in the ordered cellulose molecules which have been produced by the tree in a "disorder to order" process. In the burning, you add oxygen and get carbon dioxide and water as combustion products along with the liberated heat. The liberated heat comes from the energy stored in the ordered sugar molecules which make up the cellulose.
The process by which animals use energy is a very similar process. They too need oxygen to burn the starch in a way to produce CO2 and water with the liberation of heat. When you are burning fat or burning sugars, you are producing about the same things as if you were burning a wood fire. You do produce heat, which we warm-blooded animals use to maintain our body temperature. But we also give off a lot of heat as well. On a 24-hour average basis as a result of human energy, we may give off about the same amount of heat as a 100 watt light bulb. For living things, only about half the energy of the metabolic process is immediately released as heat - the rest is trapped in a high energy molecule which is vitally important to all the processes of life - the molecule adenosine triphosphate.
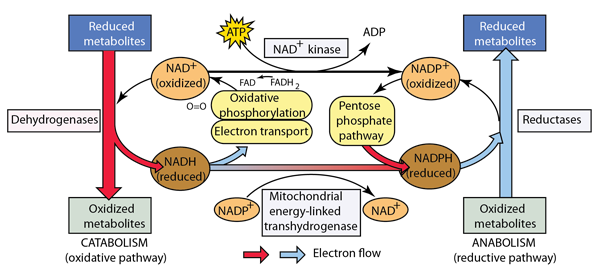
Obtaining energy from food molecule like glucose or carbohydrates involves breaking them down into smaller molecules: this general process is called catabolism. By contrast, the building up of complex molecules from simpler ones is called anabolism. The outline of the role of catabolism and anabolism below is patterned after the treatment by Matthews, van Holde, and Ahern.
| Order and disorder in biological systems. |
| Cellular Respiration |
| Oxygen required for metabolism |
Second law concepts
Matthews, van Holde, and Ahern,
Ch 12.
| HyperPhysics***** Biology | R Nave |