Reduction, Oxidation and Available Energy
Obtaining energy output from a fuel typically involves oxidation of the fuel, and if the fuel is more reduced to start with, this would imply that more energy can be obtained from it if we could fully oxidize it. Viewing a molecule that is more reduced as being in a higher energy state with respect to its totally oxidized state is useful for biological energy processes like cellular respiration.
Karp has a good example of a progression from reduced states to oxidized states of carbon compounds as a picture of how much energy might be available from molecules for metabolic processes.
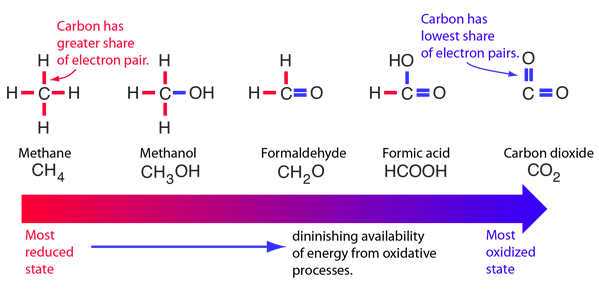
Fats represent greater energy sources than carbohydrates because they are more highly reduced.
In cell metabolism processes like glycolysis and the TCA cycle, energy from oxidation of glucose is stored by reducing the coenzymes NAD+ and FAD, providing energy for some of the cell processes.
| Electrochemistry |
Chemical concepts
Oxidation/
Reduction concepts
Reference
Karp
Ch 3
| HyperPhysics*****Chemical concepts | R Nave |