Radon in the Air
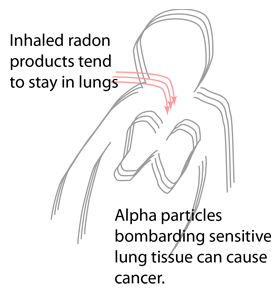
One would think that radon was the least of our radiation problems since it is an inert gas. That would be so except that when we breathe, we are constantly passing air into our lungs and out of them. In this process, the radon gas simply goes in and out, doing little damage, but the radon daughters, being basically solid materials, and sometimes being electrically charged, can stick to the surfaces of our bronchial tubes. This puts them right where they can do the most harm, for the cells lining our bronchial tubes are among the cells of our body most sensitive to radiation-induced cancer. The alpha particles emitted in the decay of radon daughters, in spite of their poor penetrating power, can reach these very sensitive cells because they are deposited so close to them. To make matters very much worse, alpha particles are much more efficient than other types of radiation for inducing cancer. The very fact that they are not penetrating means that they dump a lot of their energy into each of the biological cells they pass through, and this large release of energy into a single cell is just what is needed to initiate a cancer. As a result an alpha particle is a hundred times more likely to cause cancer than other types of radiation, if it can reach the target cells. Our breathing processes allows the alpha particles from radon daughters to reach these cells.
Radon is believed to be an important cause of lung cancer, killing about 10,000 Americans each year. Only cigarette smoking causes more lung cancer deaths per year. And in perhaps one out of a thousand American homes, radon levels are so high they pose a greater lung cancer risk than smoking a pack of cigarettes per day.
|
Index
Reference
Cohen |