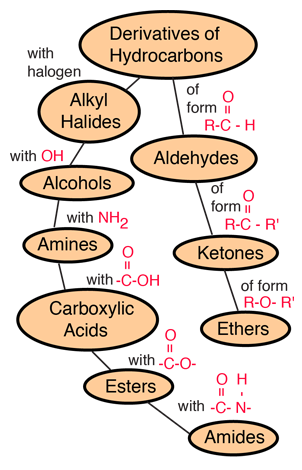
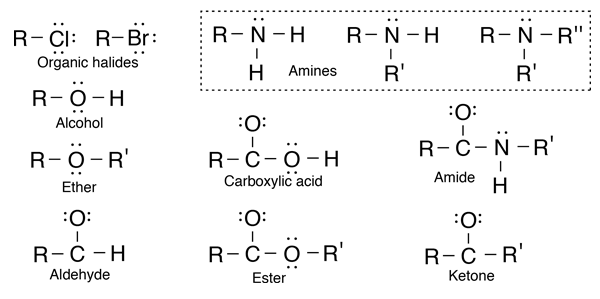
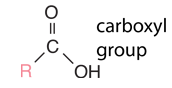
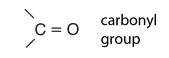Hydrocarbons
|
Hydrocarbons are the simplest organic compounds. Containing only carbon and hydrogen, they can be straight-chain, branched chain, or cyclic molecules. Carbon tends to form four bonds in a tetrahedral geometry. Hydrocarbon derivatives are formed when there is a substitution of a functional group at one or more of these positions. |
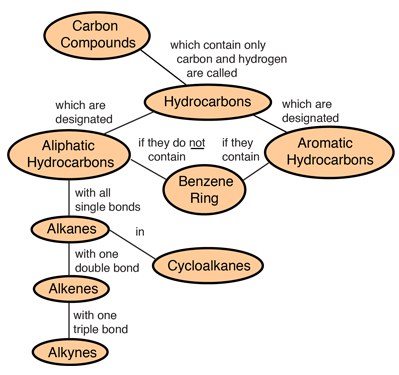
|
Organic chemistry concepts
Chemistry concepts
| HyperPhysics*****Chemistry | R Nave |