Stearic Acid
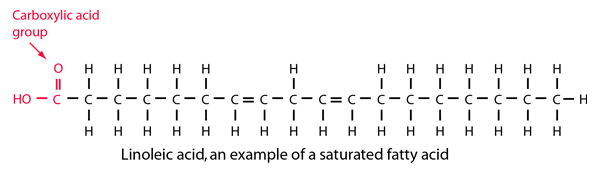
Stearic acid is a fatty acid composed of 17 carbons in a linear chain plus a carboxylic acid group. It is bonded to as many hydrogen atoms as possible, so it is said to be a saturated fatty acid.
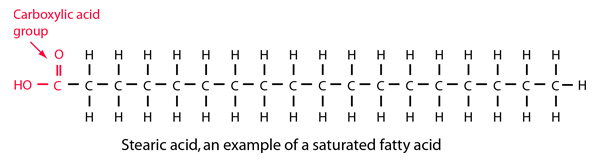
Stearic acid may be obtained from many animal and vegetable fats and oils. At room temperature it is a waxy solid.Since animal fats are typically triglycerides, stearic acid can be obtained from them by treatment with water at a high pressure and temperature to accomplish hydrolysis of the triglycerides. It can also be obtained from the hydrogenation of some unsaturated vegetable oils.
Stearic acid is used in making candles, soaps, and some plastics. It is used to harden soaps made from vegetable oils. Stearic acid compounds are used to give the pearly effect in shampoos, liquid soaps and cosmetics. Stearic acid is the source of the common scent of crayons. Further details can be found in Wikipedia, from which these details were excerpted.
Biochemical concepts
Chemistry concepts
Reference
Enger & Ross
| HyperPhysics*****Chemistry | R Nave |