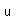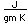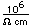Cadmium
Cadmium is a bluish-white metal. It is used as a protective coating for iron and steel. Cadmium is deposited electrolytically from a cadmium cyanide bath.
Cadmium is used in the alloy called "Wood's metal" which contains 50% Bi, 25% Pb, 12.5% Sn and 12.5% cadmium. Wood's metal melts at 65.5íC and is used for fusible links in automatic fire extinguishers.
Cadmium is very toxic, so cadmium treated metals should not be used for cooking or storing food. The fumes of zinc, cadmium and mercury are toxic.
|