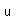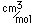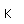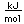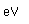Francium
Francium is the heaviest of the alkali metals and closely resembles cesium in its chemical properties. It is the most electropositive element. All its isotopes are radioactive and short-lived; its longest-lived isotope, francium-223, has a half-life of 22 minutes. It emits a beta particle of 1.1 MeV energy.
Francium is the least stable of the first 103 elements, making the measurement of its properties a considerable challenge. In nature it is found only in trace amounts in uranium deposits where it is produced in the uranium-235 decay series. In early 1996 it was manufactured by bombarding hot gold targets with oxygen-18 atoms, producing Fr-210. About 1000 of these atoms at a time were trapped in a glass chamber by the combined action of six lasers at 718 nm and a magnetic field.
The fluorescence from a collection of about 10,000 such atoms was bright enough to be recorded by a video camera. Further work should yield details about its atomic structure.
Interest in francium comes from the theoretical indications tha its heavy, unstable nucleus would provide a sensitive test for electron-quark interactions (weak interaction).
|