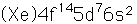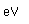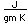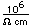Iridium
Iridium is a metal of the platinum group. It is very unreactive and is sometimes alloyed with platinum to form a very hard alloy. Platinum-iridium is used for the tips of gold pens, and for surgical tools.
Iridium was brought into public discussion with the discovery of an iridium-rich layer in the strata of soil and rock which covers the entire planet. It is part of the evidence for an asteroid collision with the earth, widely associated with the extinction of the dinosaurs about 65 million years ago.
|