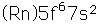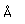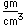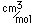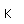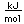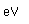Plutonium
Plutonium is a man-made trans-uranic element. Plutonium-238 was the first isotope to be made. The process involved bombarding uranium-238 with deuterium. Neptunium-239 is made as an intermediate product, which then decays to form plutonium-238.
Plutonium was first discovered by Glenn Seaborg and collaborators at the University of California, Berkeley.
| Plutonium-238 is used as the main heat source for Radioisotope Thermal Generators which supply electric power for spacecraft. It's relatively short half-life makes it a potent heat source. At right shows the glowing of a pellet of PuO2 from its own self-heating ( from Wikipedia). |  |
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 29 |