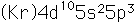Antimony
Antimony is a brittle metal, silvery gray in color. It has the property of expanding upon freezing, and its main application has been as a constituent of type metal (82% lead, 15% antimony, 3% tin). The expansion upon solidifying gives sharp reproduction of type characters in the molds. Antimony is also used as a constituent of other alloys for uses like the grids in storage batteries and for bearings. One mineral source of antimony is its sulfide contained in the mineral stibnite.
Antimony forms multiple sulfide minerals. Antimony forms the sulfide kermesite, Sb2OS2.
Antimony forms a sulfide with lead and iron called Jamesonite. A sulfide of antimony along with copper and iron is called tetrahedrite. A sulfide with iron forms the mineral berthierite and with copper the mineral chalcostibite, CuSbS2. Antimony forms sulfides with lead called zinkenite, plagionite, semseyite and boulangerite. It also forms sulfides with silver called Stephanite, miargyrite and pyrargyrite. The sulfide formed with nickel is called ullmannite. A sulfide formed with lead and arsenic is called jordanite. A sulfide formed with tin, iron and lead is called cylindrite. A sulfide formed with tin and lead is called franckeite. Bournonite is a sulfide with copper, lead and antimony. Tennantite is a sulfide which contains antimony, copper, iron and arsenic.
Antimony is found in the mineral nagyagite, a sulfide, along with lead, iron, gold, and tellurium. Silver joins with lead and antimony in the sulfide andorite, AgPbSb3S6. Antimony with copper and silver form the sulfide polybasite.
Antimony oxide, Sb2O3, forms the minerals valentinite and senarmontite. An oxide of antimony and tantalum is stibiotantalite. Antimony and titanium are contained in the oxide romeite.
Stibarsen, SbAs, is a silvery mineral with a metallic luster.
In the mineral dyscrasite, silver bonds directly to antimony in the form Ag3Sb.
Antimony is found in the silicate mineral Langbanite along with manganese, iron and calcium.
A chloride of lead and antimony is nadorite, PbSbO2Cl.

|
Antimony is one of the few elements which can be found in nature in pure form. This sample of antimony is displayed in the Smithsonian Museum of Natural History. The sample is about 4x5 cm and is from Consols mine, Broken Hill, New South Wales, Australia.
|
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 16, 28 |