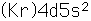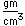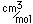Yttrium
Yttrium forms colorless compounds similar to those of aluminum. It had found few applications until it contributed to the study of superconductivity by being a part of the yttrium-barium-copper oxide superconductors. It is usually found in nature with scandium and lanthanum and the fourteen members of the lanthanide series.
Yttrium forms a phosphate YPO4 in the mineral xenotime. Yttrium is found in the minerals aeschynite, euxenite, bastnaesite and gadolinite. It appears with scandium in the silicate mineral Thortveitite. It appears with cerium and uranium in samarskite.
|