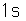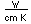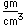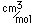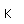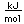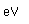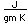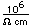Hydrogen
Hydrogen is the lightest and most abundant element in the universe. The hydrogen-helium abundance ratio is an important clue to the cosmological process. The nucleus of ordinary hydrogen consists of a single proton, but it also occurs as deuterium with a proton and a neutron. Deuterium as an isotope of hydrogen has an abundance of 1.5 x 10-4 compared to 0.99985 for ordinary hydrogen. It's stability is remarkable since the free neutron is unstable, undergoing beta decay with a halflife of 10.3 minutes. The measured binding energy of the deuteron is 2.2 MeV. Hydrogen also exists as tritium with a proton and two neutrons but is unstable with a halflife of 12.32 years.
Hydrogen is found in most of the substances which constitute living matter. There are more compounds of hydrogen known than any other element, the most important being water.
The lightest of the gases, hydrogen's density is one-fourteenth that of air. It's melting point (14 K) and boiling point (20.3 K) are lower than any other element except helium. As might be expected, liquid hydrogen at a density of 0.07 gm/cm3 is the lightest of all liquids. Crystalline solid hydrogen at 0.088 gm/cm3 is the lightest of all crystalline substances (lighter than a marshmallow!).
Large amounts of hydrogen are used in industry in converting oils (liquid fats) into solid fats for the production of foodstuffs and soaps. This process is called hydrogenization.
Hydrogen has a higher thermal conductivity and lower viscosity than other gases and is sometimes used in closed systems for the rapid removal of heat. It has been used as a cooling gas around the armatures of large electric generators.
Hydrogen burns in oxygen or air with an almost colorless flame and has potential as a fuel. Since it requires energy to separate hycrogen, its real potential is as a storage medium for energy; processes like electrolysis of water can produce hydrogen for storage as a fuel.
Hydrogen is in the same vertical column as the alkali metals, but under conditions occurring on the earth it is a gas. When extremely compressed, hydrogen takes on the characteristics of a liquid metal. The deep interior regions of Jupiter and Saturn produce the pressure conditions to produce this liquid metallic state.
|