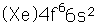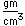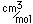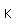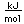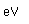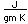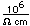Samarium
As a member of the fourteen member lanthanide series, this element has few properties which distinguish it from the other members of the series. All of them along with lanthanum, yttrium, and scandium occur in very small quantities in nature. The usual source is the mineral monazite, or monazite sand, which is a mixture of phosphates containing also some thorium phosphate.
Samarium has found recent use in the manufacture of rare earth magnets which produce very high magnetic field strengths with small masses.
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 26 |