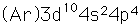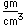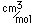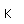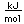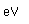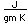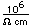Selenium
Selenium occurs as a gray material and as a red allotropic material. The gray material has a small electrical conductivity in the dark which increases dramatically with exposure to light. This property is used in "selenium cells" for light sensors for control applications.
Selenium is used to impart a ruby-red color to glass and to neutralize the green color in glass which is present due to iron content.
Selenium is similar to sulfur in its chemical properties. One mineral form of selenium is the compound with lead, clausthalite, PbSe.
|