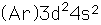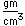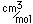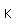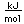Titanium
Titanium is a transition metal which is very strong and light (4.4 gm/cm3). It has a high melting point (1800°C) and finds application in aircraft wings, particularly at points which are subjected to the high heat of exhaust.
Titanium dioxide in powder form is very white and is used to advantage as a pigment in special paints and face powders. Titanium dioxide, TiO2, in crystalline form appears in the mineral rutile. It may be colored by small amounts of other metal oxides and has been used for gems. Titanium dioxide also makes up the minerals Brookite and anatase. Titanium and iron together form the oxides ilmenite, FeTiO3, and pseudobrookite, Fe2TiO5. Titanium and calcium form the oxide perovskite, CaTiO3. Titanium and calcium form the silicate titanite, CaTiSiO5, which can form gem-quality crystals. Lithium and sodium are found with either zirconium, titanium or hafnium in the mineral zektzerite. Titanium appears with manganese and arsenic in the mineral cafarsite
Titanium is found in the minerals aeschynite, euxenite and
astrophyllite. Titanium forms an oxide mineral with lead, iron and manganese called senaite. Another oxide with boron (borate) with iron and titanium is warwickite. Antimony and titanium are contained in the oxide romeite. Titanium is found in the silicate minerals joaquinite, wilhuite and neptunite.
Titanium tetrachloride is a liquid at room temperature, but if it is sprayed into the air, it hydrolizes to form HCl and fine particles of titanium dioxide. It has been used for making smoke screens. A spray of titanium tetrachloride is used for "skywriting" from aircraft.
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 29 |