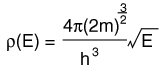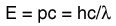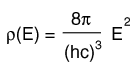The Distribution of Energy
The distribution of a fixed amount of energy among a number of identical particles depends upon the density of available energy states and the probability that a given state will be occupied. The probability that a given energy state will be occupied is given by the distribution function, but if there are more available energy states in a given energy interval, then that will give a greater weight to the probability for that energy interval.
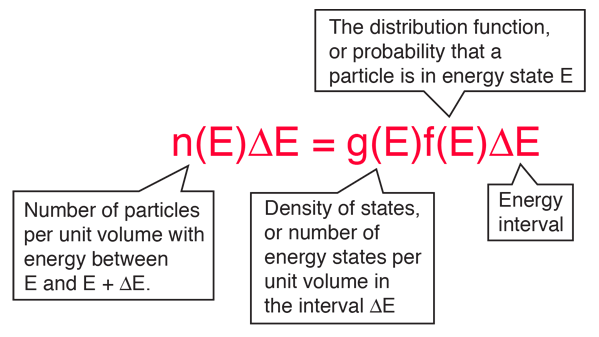
| The distribution function | The density of states |
Applied statistics concepts
| HyperPhysics***** Quantum Physics | R Nave |