Sublimation
Sublimation is the term for a phase change that proceeds directly from the solid phase to the vapor phase without first making the transition to the liquid state. The most common example of sublimation is that of solid carbon dioxide, CO2, or "dry ice". At ordinary temperatures and pressures on the Earth, most pure substances must pass through a liquid phase when the solid is heated before becoming a gas.
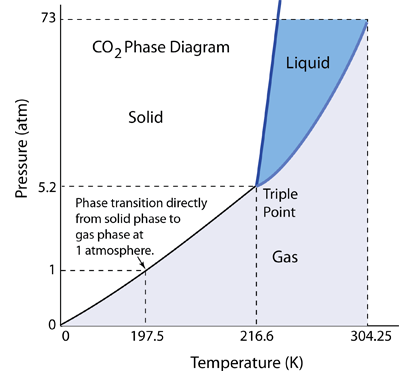 | The conditions under which solid, liquid and gaseous phases exist may be summarized on a Pressure vs Temperature diagram, or more generally on a PvT surface. The phase diagram for CO2 at left shows a triple point at which all three phases can exist, but it is at 5.2 atmospheres. If the solid CO2 is heated at one atmosphere pressure, it "sublimes" or makes the transition directly from the solid to the gas phase. |
| Water phase changes |
Phase change concepts
John Denker
| HyperPhysics***** Thermodynamics | R Nave |