Acids and Bases: Titration
 |  |
The acid and base character of common products was to be investigated. Standard solutions of known pH had been prepared.
 |  |
Red cabbage juice was one of the indicators used.
 |  |
Samples included grape juice, beet juice, and vinegar above left. Samples were placed in individual compartments for testing.
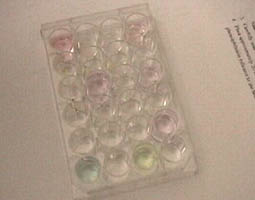 |  |
With indicators, the pH of the samples could be bracketed.
 |  |
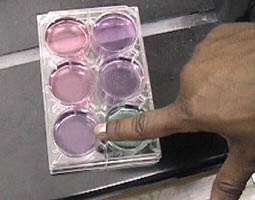
For more precise pH determination, titration was to be used. Dr. Ellen Verdel demonstrates the use of the pipete and describes the process.
 |  |
The point of completion of the titration process had to be approached carefully. A drop of the standard solution would turn the sample pink at the entry point, but would clear with stirring. At right above is a carefully determined final point with just a hint of pink showing.