Experimental methods for obtaining atomic spectra
The study of atomic energy levels is accomplished by observing transitions between electron energy levels in atomic spectra.
 | One of the most straightforward ways to obtain atomic line spectra is to electrically excite a gas with a high voltage and observe the emitted light through a diffraction grating. |
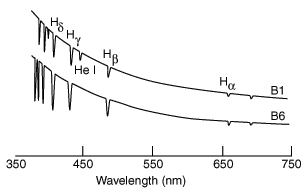 | The same kind of spectral data can be obtained by measuring atomic absorption, since the same quantum energy levels are involved. This allows us to identify chemical elements in distant stars by measuring their spectral fingerprints in the absorption spectra of the star's light. |
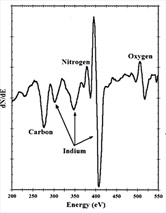 | Another type of spectroscopy makes use of the Auger effect which ejects electrons from atoms in response to electron transitions. The measured energy of the ejected electrons gives information about atomic energy levels and permits identification of the atoms, even in the solid state. |
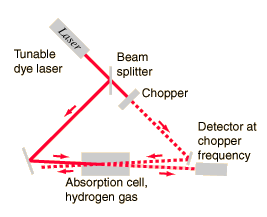 | Saturation spectroscopy using tunable laser sources is a method for obtaining extremely high resolution spectra. |
| Thwarting Doppler broadening with saturation spectroscopy |
Atomic Structure Concepts
| HyperPhysics***** Quantum Physics | R Nave |