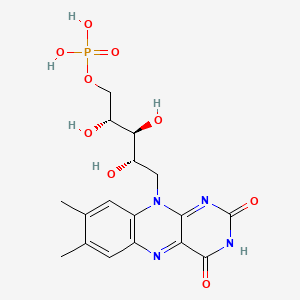
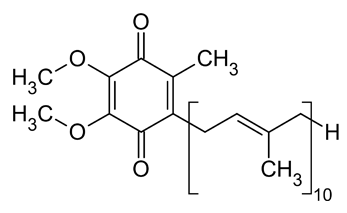
NADH-Coenzyme Q oxidoreductase (Complex I)
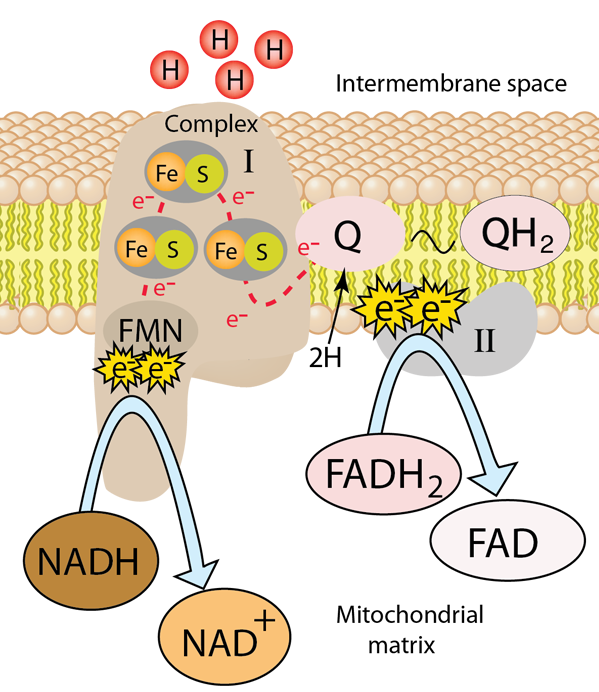
The first protein complex in the electron transport chain is named NADH-Coenzyme Q oxidoreductase and is commonly labeled Complex I. It is also called NADH dehydrogenase. It is a large L-shaped multiunit protein complex which accepts the high energy electrons from NADH. The NADH molecule donates two electrons to an acceptor group flavin mononucleotide (FMN) which is found on the vertical arm of the complex as shown below. The FMN is reduced to the form FMNH2.
From this point the electrons move along a series of iron-sulfur groups (about eight of them) and are transferred to the associated coenzyme Q (ubiquinone). The ubiquinone also extracts two protons from the matrix to form the fully reduced ubiquinol (QH2). As the electrons are moving through the series of FeS clusters, they use the provided electrical energy to pump 4 H+ ions out of the mitochondrial matrix and into the intermembrane space to provide them for the production of the high energy molecule ATP in the oxidative phosphorylation process.
| to Complex II |
Photosynthesis Concepts
Reference
Moore, et al.
Ch 7
Ahern
Biochemistry.., Ch14
Karp
Ch 5.3
AK Lectures
Complex I & II
| HyperPhysics***** Biology | R Nave |