Alanine
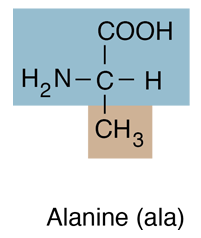 | Alanine is an amino acid with a methyl group in the R position and belongs to the class which has hydrocarbon R-groups. Amino acids of this group are generally hydrophobic, and tend to contribute to closeness in protein folding as they gather together to avoid water. |
Alanine is abundant in protein structures, being second only to leucine in rate of occurrence. It accounts for 7.8% of the primary structure in a sample of 1,150 proteins. (wiki) Its chirality is the L-form in humans, but it is one of the rare amino acids that does occur in the right-handed or D-form in some bacterial cell walls and in the tissues of many crustaceans and molluscs.
"In mammals, alanine plays a key role in glucose-alanine cycle between tissues and liver. In muscle and other tissues that degrade amino acids for fuel, amino groups are collected in the form of glutamate by transamination. Glutamate can then transfer its amino group to pyruvate, a product of muscle glycolysis, through the action of alanine aminotransferase, forming alanine and alpha-ketoglutarate. The alanine enters the bloodstream, and is transported to the liver. The alanine aminotransferase reaction takes place in reverse in the liver, where the regenerated pyruvate is used in gluconeogenesis, forming glucose which returns to the muscles through the circulation system. Glutamate in the liver enters mitochondria and is broken down by glutamate dehydrogenase into alpha-ketoglutarate and ammonium, which in turn participates in the urea cycle to form urea which is excreted through the kidneys."
"The glucose-alanine cycle enables pyruvate and glutamate to be removed from muscle and safely transported to the liver. Once there, pyruvate is used to regenerate glucose, after which the glucose returns to muscle to be metabolized for energy: this moves the energetic burden of gluconeogenesis to the liver instead of the muscle, and all available ATP in the muscle can be devoted to muscle contraction." (Wiki)
| Alanine wiki |
Biochemical concepts
Chemistry concepts
Reference
Tillery, Enger and Ross
Ch 14
Ahern
| HyperPhysics*****Chemistry *****Organic Chemistry | R Nave |