Serine
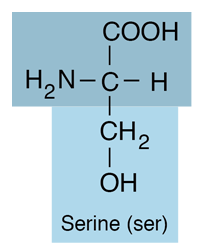 | Serine is an amino acid and belongs to the class which has neutral R-groups. It has a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It is hydrophilic. |
"Serine is important in metabolism in that it participates in the biosynthesis of purines and pyrimidines. It is the precursor to several amino acids including glycine and cysteine, as well as tryptophan in bacteria. It is also the precursor to numerous other metabolites, including sphingolipids and folate, which is the principal donor of one-carbon fragments in biosynthesis."
"Serine plays an important role in the catalytic function of many enzymes. It has been shown to occur in the active sites of chymotrypsin, trypsin, and many other enzymes. The so-called nerve gases and many substances used in insecticides have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely."
"It is one of three amino acid residues that are commonly phosphorylated by kinases during cell signaling in eukaryotes. Phosphorylated serine residues are often referred to as phosphoserine."
Serine proteases are a common type of protease.
"Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, sericum. Serine's structure was established in 1902. Food sources with high L-Serine content among their proteins include eggs, edamame, lamb, liver, pork, salmon, sardines, seaweed, tofu."
| Serine wiki |
Biochemical concepts
Chemistry concepts
Reference
Tillery, Enger and Ross
Ch 14
Ahern
| HyperPhysics*****Chemistry *****Organic Chemistry | R Nave |