Cytochrome C Oxidase (Complex IV)
The final protein complex in the electron transport chain is named cytochrome C oxidase and is commonly labeled Complex IV. It is a large collection of polypeptides arranged in 13 subunits, three of which are encoded in the mitochondrial genome. The electron carrier cytochrome c transfers a single electron, but multiple electrons are required for handling oxygen, so the process is complicated and has required intensive study. The electrons from cytochrome c are first provided to a complex with two atoms of copper metal (CuA), then transferred to the first of two heme groups (heme a) with iron metal. The next transfers are to a second heme group(heme a3) and then shared with a third copper atom (CuB).
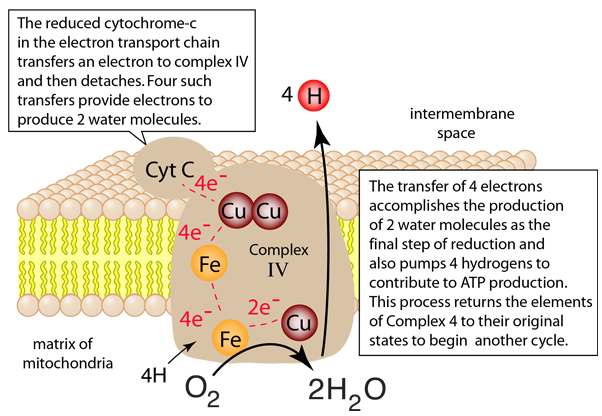
The process in Complex IV which reduces an oxygen molecule to a water molecule and providing 4 hydrogens to the intermembrane space is summarized in four steps below. Electrons are transferred one at a time from cytochrome c through the copper center to the first iron-containing heme group. From there they are transferred to the second heme group and the third copper atom. When the first two electrons are received, an oxygen molecule can bind to the center, but the double bond is broken to form an energetic peroxide bridge (step 2). The arrival of two more cytochrome c provides two more electrons and the extraction of two hydrogens from the matrix leads to forming two hydroxides (step 3). The entry of two additional protons from the matrix leads to the formation of two molecules of water (step 4).
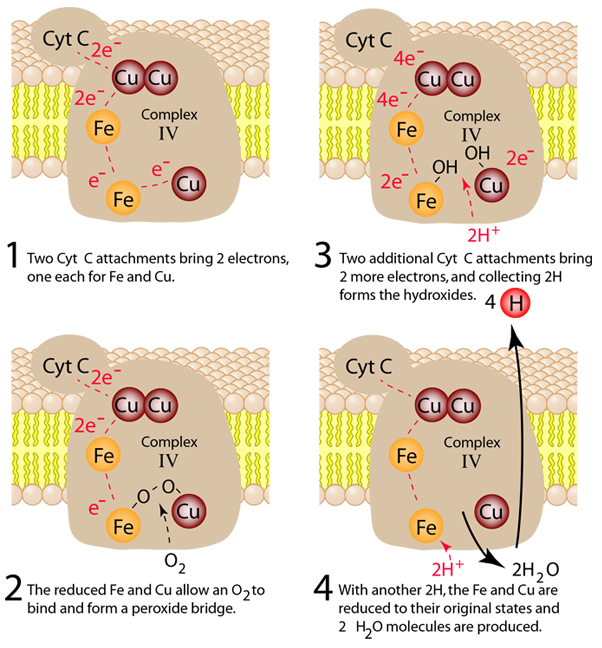
Note that these four steps leave the complexes in their original states so that the process can be repeated. This is a redox process which in addition to the reduction of the oxygen to water acts as a proton pump to extract 8 protons from the matrix and deliver 4 of them to the intermembrane space where they can contribute to the process of oxidative phosphorylation to produce the high energy molecule ATP.
Photosynthesis Concepts
Reference
Moore, et al.
Ch 7
Ahern
Biochemistry.., Ch14
Karp
Ch 5.3
| HyperPhysics***** Biology | R Nave |