Chemical Elements for Life
The chemical elements most prominent in building the structures of the molecules of life are carbon, hydrogen, oxygen, nitrogen, phosphorous and sulfur, sometimes represented mnemonically as CHONPS. These elements combine in a vast number of molecules involved in life processes. Their fitness for making these compounds is related to their relative electronegativities which contribute to their ease of combination.
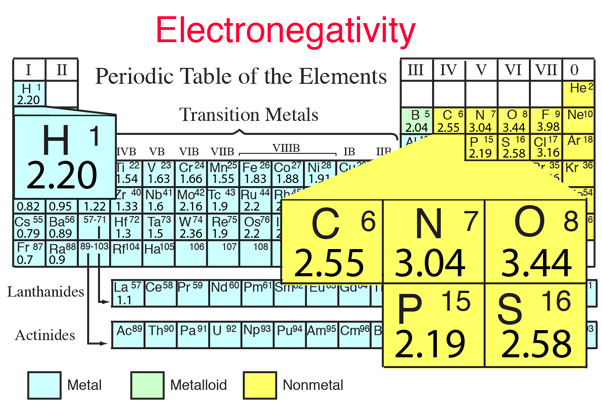
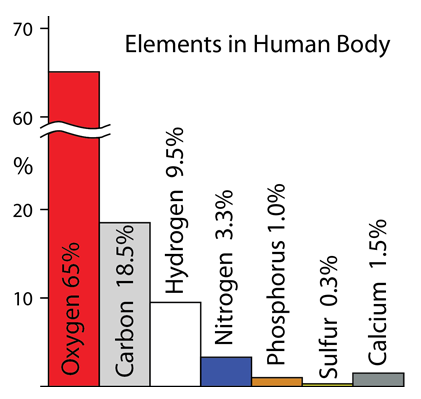 | These elements make up the bulk of the atoms in living organisms. The CHONPS elements are the most abundant in the body with the exception of calcium at 1.5%. In addition to these elements, a number of metal atoms play essential supporting roles in producing enzymes, contributing to communication pathways, and enabling specific processes in the body. |
Carbon: Carbon is the foundational element for all life on Earth. It's capacity to form four bonds makes possible an almost limitless variety of compounds.
- Hydrocarbons contain only carbon and hydrogen.
- Derivatives of Hydrocarbons add other elements to carbon and hydrogen.
- Aromatic Hydrocarbons contain one or more benzine rings.
Oxygen: Oxygen is the most abundant element in the human body at 65%. It is abundant partly because the body is up to 60% water, but is also a constituent of most large biological molecules and is continually being exchanged in the process of metabolism and respiration.
- Derivatives of Hydrocarbons mostly have oxygen as a constituent in addition to carbon and hydrogen.
- Respiration continuously transports oxygen to cellular processes.
- Cellular Respiration oxidizes food in the metabolic process for energy.
- Energy production in the electron transport chain makes ATP and forms water for removal.
Hydrogen: Hydrogen with oxygen is abundant in the body because it is up to 60% water.
- Hydrocarbons and their derivatives contain carbon and hydrogen.
- Energy production in the electron transport chain transports hydrogen for making ATP .
Nitrogen: Nitrogen is not abundant on the Earth, but is crucial in life processes.
- Amino acids are the building blocks for proteins.
- Nucleotides which make up nucleic acids are essential to life.
- DNA is the master storage molecule for information for building proteins.
- RNA is directly involved in transcribing information for building proteins and other parts of the translation process of building proteins.
Phosphorus: Phosphorus is not so abundant, but is crucial in the energy sources for life processes
- ATP is the phosphate compound which is the basic energy source for most molecular interactions in life.
- Glycolysis uses ATP and NAD+ to convert glucose to pyruvate in the energy chain.
Sulfur: Sulfur has many structural roles in proteins.
- Amino acids cysteine and methionine contain sulfur and disulfide bonds in protein folding accomplish key tasks.
Calcium: Calcium is a key component of bones and calcium ions also handle information in neural processes. As a chemical messenger, it has not only high speed but also the ability to bind to another molecule in the cell like a protein with high specificity to enact a conformational change. Calcium and magnesium can accomplish this, but calcium is superior for the role, binding many times more strongly than magnesium.
- Muscle fiber contraction is initiated by information transported by calcium ions.
The Role of Metal Atoms in Body Chemistry
Although present in relatively small amounts, metal atoms play a surprisingly important role in the chemistry of life. About a third of the enzymes in the body involve a metal ion as an essential participant. From the electron transport chain to the maintaining of membrane potentials of cells, metals play essential supporting roles for the atoms involved in the major structures of life.

Sodium: Sodium binds weakly to organic compounds and sodium ions are small and highly mobile, ideally suited for moving electric charge at high speeds. Sodium and potassium are the only ions whose speed is unimpeded by a tendency to bind to organic compounds.
- Cell membrane transport: The ease and speed of movement across cell membranes helps to maintain proper electrical potentials. Sodium participates in active transport processes across cell membranes.
- Action potentials: The mobility of sodium ions across membranes makes them useful for transmission of nerve impulses associated with nerve cells.
Magnesium is present in every cell type in every organism. Magnesium enyzmes are necessary for the catalytic action of more than 300 enzymes.
- Mg2+-ATP association: ATP, the main provider of cellular energy, is always associated with magnesium.
- Photosynthesis: Magnesium is a key component of chlorophyll and is essential to the efficiency of capturing light energy. Magnesium has been found to be far more efficient than any other metal ion, sometimes by a factor of thousands.
Potassium: Potassium binds weakly to organic compounds and potassium ions are small and highly mobile, ideally suited for moving electric charge at high speeds. Potassium and sodium are the only ions whose speed is unimpeded by a tendency to bind to organic compounds.
- Cell membrane transport: The ease and speed of movement across cell membranes helps to maintain proper electrical potentials. Potassium participates in active transport processes across cell membranes.
- Action potentials: The mobility of potassium ions across membranes makes them useful for transmission of nerve impulses associated with nerve cells.
Manganese: Manganese is important in plant photosynthesis.
- Splitting water in green plants is part of photosynthesis in photosystem-II. It involves manganese chemical reactions.
Iron: One of the most important metals in biological processes, iron is a key to oxygen transport by hemoglobin, to electron transport on the way to ATP production, and part of a catalyst for the fixation of nitrogen in plants.
-
Hemoglobin: Iron is a key component of hemoglobin, which transports oxygen to the cells inside red blood cells.
- Nitrogen fixation in the roots of plants is an essential process for life. An enzyme containing iron and molybdenum is used in the process of making NH3 from N2.
- Electron transport chain: Iron and copper are key constituents of Complex IV in the electron transport chain. They accomplish the transfer of electrons to oxygen to prepare for the final step of making a water molecule, and also transfer hydrogen ions to the intermembrane space of the mitochondria for the process of oxidative phosphorylation and making of ATP. Iron in the form of Fe-S complexes is a part of Complex I, Complex II, and Complex III of the electron transport chain as parts of the pathway of conducting the electrons through the chain.
Cobalt: Cobalt and nickel are sometimes referred to as evolutionary relics because of their frequent appearance in ancient anaerobic bacteria. Vitamin B12 has a single atom of cobalt. Current uses involve vitamin B12-dependent enzymes.
- Vitamin B12 is associated with a number of enzymes which contain cobalt. It is a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. It is important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the maturation of red blood cells in the bone marrow. One application is in the synthesis of high fructose corn syrup. In this and other cobalt applications, other metals may be substituted.
Copper: A key element in the electron transport chain which leads to the energy molecule ATP in the cell.
- Electron transport chain: Copper and iron are key constituents of Complex IV in the electron transport chain. They accomplish the transfer of electrons to oxygen to prepare for the final step of making a water molecule, and also transfer hydrogen ions to the intermembrane space of the mitochondria for the process of oxidative phosphorylation and making of ATP.
Zinc: There is typically only about 3 grams of Zinc in the body, but it is present in over 300 enzymes. It has a crucial role in converting CO2 to H2CO3 for transport to the lungs.
- Carbonic Anhydrase is an enzyme with zinc as a key component. It is involved in converting the metabolic product CO2 to carbonic acid, H2CO3, in the red blood cells for transport to the lungs. When red blood cells reach the lungs, the same enzyme helps to convert the bicarbonate ions back to carbon dioxide, which we breathe out.
Molybdenum: Molybdenum is the key metal ion component in four important enzymes in our bodies. It is the key metal ion involved in nitrogen fixation in plants.
- Nitrogen fixation in the roots of plants is an essential process for life. An enzyme containing molybdenum is used in the process of making NH3 from N2.
Chemistry of elements
References:
Denton Miracle of the Cell, Ch 6
Crichton
| HyperPhysics***** Chemistry | R Nave |