Comparison of Properties of Ionic and Covalent Compounds
Because of the nature of ionic and covalent bonds, the materials produced by those bonds tend to have quite different macroscopic properties. The atoms of covalent materials are bound tightly to each other in stable molecules, but those molecules are generally not very strongly attracted to other molecules in the material. On the other hand, the atoms (ions) in ionic materials show strong attractions to other ions in their vicinity. This generally leads to low melting points for covalent solids, and high melting points for ionic solids. For example, the molecule carbon tetrachloride is a non-polar covalent molecule, CCl4. It's melting point is -23°C. By contrast, the ionic solid NaCl has a melting point of 800°C.
Ionic Compounds
| Covalent Compounds
|
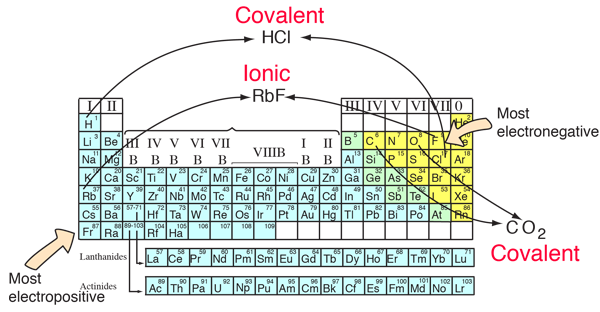
You can anticipate some things about bonds from the positions of the constituents in the periodic table. Elements from opposite ends of the periodic table will generally form ionic bonds. They will have large differences in electronegativity and will usually form positive and negative ions. The elements with the largest electronegativities are in the upper right of the periodic table, and the elements with the smallest electronegativities are on the bottom left. If these extremes are combined, such as in RbF, the dissociation energy is large. As can be seen from the illustration below, hydrogen is the exception to that rule, forming covalent bonds.
Elements which are close together in electronegativity tend to form covalent bonds and can exist as stable free molecules. Carbon dioxide is a common example.
Bond concepts
Chemical concepts
Reference
Shipman, Wilson, Todd
Ch 12
| HyperPhysics***** Quantum Physics ***** Chemistry | R Nave |