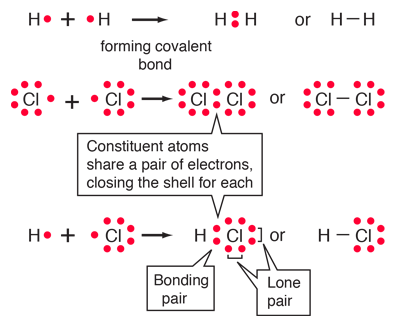
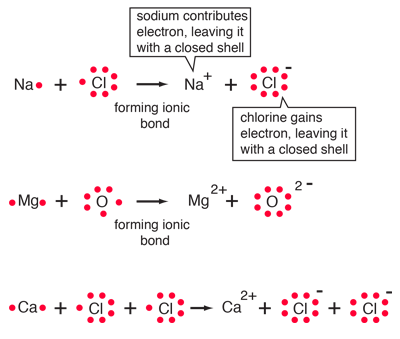
Chemical Bonding
Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms ... a chemical bond. The two extreme cases of chemical bonds are:
Covalent bond: bond in which one or more pairs of electrons are shared by two atoms.
Ionic bond: bond in which one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other.
Other types of bonds include metallic bonds and hydrogen bonding. The attractive forces between molecules in a liquid can be characterized as van der Waals bonds.
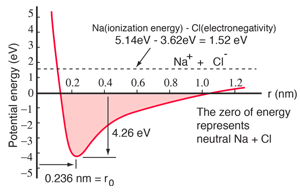 Sodium chloride Ionic | 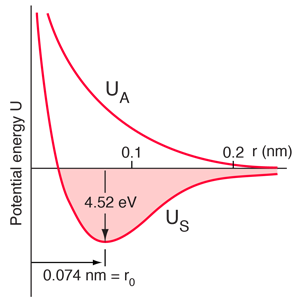 Hydrogen molecule Covalent |
Bond concepts
Bond data
Chemical concepts
| HyperPhysics***** Quantum Physics ***** Chemistry | R Nave |