Lewis Diagrams for Compound Formation
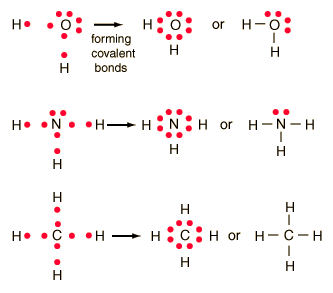
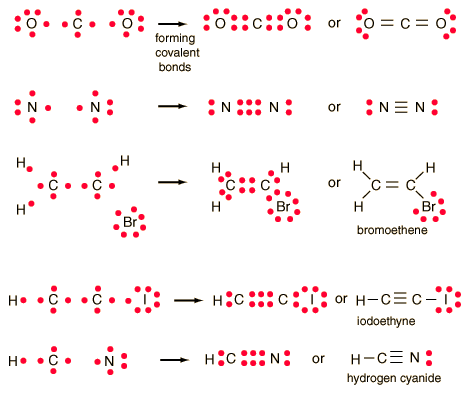
The formation of many common compounds can be visualized with the use of Lewis symbols and Lewis diagrams. In a Lewis symbol, the inner closed shells of electrons can be considered as included in chemical symbol for the element, and the outer shell or valence electrons are represented by dots. The dots are placed in four groups of one or two electrons each, with 8 electrons representing a closed shell or noble gas configuration. Lewis diagrams are useful for visualizing both ionic and covalent bonds.
In the idealized ionic bond, one atom gives up an electron to the other, forming positive and negative ions.
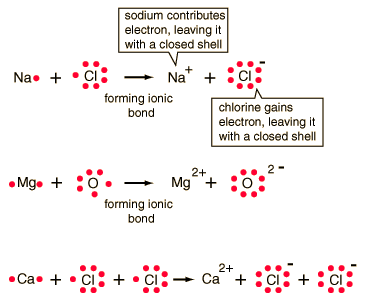 | The conditions for bonds are that the total charge is zero and that each atom must have a noble gas electron configuration. |
In the idealized covalent bond, two atoms share a pair of electrons, closing the shell for each of them.
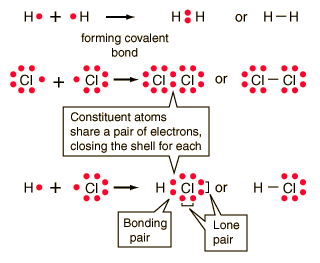 | The atoms share a pair of electrons, and that pair is referred to as a bonding pair. The pairs of electrons which do not participate in the bond have traditionally been called "lone pairs". A single bond can be represented by the two dots of the bonding pair, or by a single line which represents that pair. The single line representation for a bond is commonly used in drawing Lewis structures for molecules. |
Chemistry concepts
Reference
Shipman, Wilson, Todd
Ch 12
| HyperPhysics | R Nave |