Electrode Potentials and Equilibrium Constants
The cell potential of a voltaic cell is a measure of the maximum amount of energy per unit charge which is available to do work when charge is transferred through an external circuit. This maximum work is equal to the change in Gibbs free energy, ΔG, in the reaction. These relationships can be expressed as
Maximum work = ΔG = -nFE°cell
where n is the number of electrons transferred per mole and F is the Faraday constant.
This free energy change can also be related to the equilibrium constant K
ΔG = -RT ln K
Combining these relationships allows us to express the cell potential in terms of the equilibrium constant.
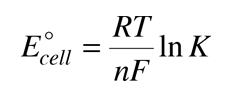
Consider the historic Daniell cell in which zinc and copper were used as electrodes. The data from the table of standard electrode potentials is
| Cathode (Reduction) Half-Reaction | Standard Potential E° (volts) |
| Zn2+(aq) + 2e- → Zn(s) | |
| Cu2+(aq) + 2e- → Cu(s) |
The standard cell potential is then E°cell = 1.1 volt and 2 electrons are transferred per mole of reactant. The relationship for the equilibrium constant is then

This extremely high value for the equilibrium constant confirms that the reaction of the Daniell cell is indeed spontaneous and that it will proceed until the reactants are exhausted.
| Table of Standard Electrode Potentials |
Oxidation/
Reduction concepts
Electrochemistry concepts
Reference
Hill & Kolb
Ch 8
Ebbing
Ch 19
| HyperPhysics***** Electricity and Magnetism ***** Chemistry | R Nave |