Ionization Energies
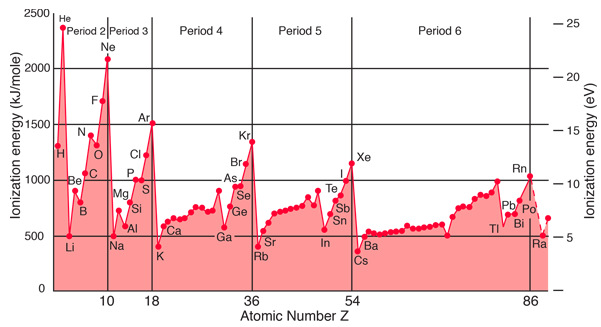
The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom. It is a minimum for the alkali metals which have a single electron outside a closed shell. It generally increases across a row on the periodic maximum for the noble gases which have closed shells. For example, sodium requires only 496 kJ/mol or 5.14 eV/atom to ionize it while neon, the noble gas immediately preceding it in the periodic table, requires 2081 kJ/mol or 21.56 eV/atom. The ionization energy is one of the primary energy considerations used in quantifying chemical bonds.
The ionization energy for any element may be found by clicking on its chemical symbol in the periodic table.
Chemical bond concepts
Reference
Ebbing
| HyperPhysics***** Chemistry | R Nave |