Viscosity of Gases
The viscosity of a gas can be thought of as a measure of its resistance to flow and is measured in the CGS unit Poise = dyne sec/cm2. The viscosity of gases near room temperature are in the centiPoise range, so that is a commonly used unit. Gas viscosity is only weakly dependent on pressure near atmospheric pressure. It is primarily a function of temperature, and can be modeled in terms of temperature with the input of experimental reference measurements. Note that as an engineering quantity, the temperatures used are in the Rankine scale.
Gas viscosity can be modeled by Sutherland's formula:
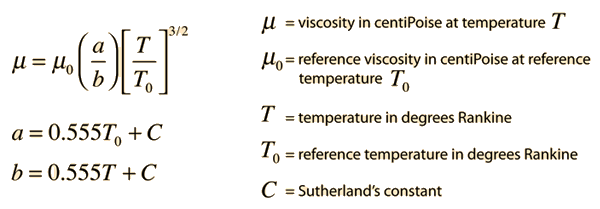
Since Sutherland's formula is an empirical fit of measured data, the following table of reference data is needed.
| Gas | Sutherland's constant C | °R | centiPoise |
| standard air | |||
| ammonia, NH3 | |||
| carbon dioxide, CO2 | |||
| carbon monoxide, CO | |||
| hydrogen, H2 | |||
| nitrogen, N2 | |||
| oxygen, O2 | |||
| sulfur dioxide, SO2 |
References:
For gas viscosity: Chemical Rubber Company (CRC). 1984. CRC Handbook of Chemistry and Physics. Weast, Robert C., editor. 65th edition. CRC Press, Inc. Boca Raton, Florida. USA.
For Sutherland's formula and values for : Crane Company. 1988. Flow of fluids through valves, fittings, and pipe. Technical Paper No. 410 (TP 410)
| Mean free path related to viscosity |
Gas law concepts
Kinetic theory concepts
| HyperPhysics***** Thermodynamics | R Nave |