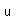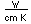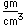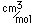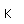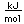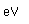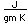Neon
Neon is the second noble gas. It represents the closing of the n=2 shell of electrons. It is found in the atmosphere at a concentration of about 0.002% . It is isolated mainly by the distillation of liquid air.
The brilliant emission from low pressure neon gas when it is excited by an electric discharge has made it very popular for illuminated signs. The term "neon sign" is commonly used for all such luminous gas signs, but the neon produces only the bright orange characteristic color. The other colors come from different gases such as helium, argon, mercury and combinations with each other and with neon.
|
Index
Periodic Table
Chemistry concepts
Reference
Pauling
Ch. 5 |