Temperature
A convenient operational definition of temperature is that it is a measure of the average translational kinetic energy associated with the disordered microscopic motion of atoms and molecules. The flow of heat is from a high temperature region toward a lower temperature region. The details of the relationship to molecular motion are described in kinetic theory.The temperature defined from kinetic theory is called the kinetic temperature. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so two objects with the same temperature do not in general have the same internal energy (see water-metal example). Temperatures are measured in one of the three standard temperature scales (Celsius, Kelvin, and Fahrenheit).
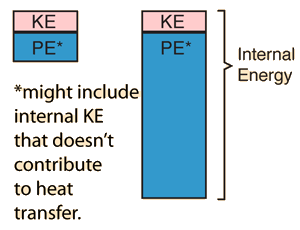
| More generalized view of temperature |
Temperature concepts
| HyperPhysics***** Thermodynamics | R Nave |
